
Benzyl 6-Bromospiro[Indoline-3,4'-Piperidine]-1'-Carboxylate synthesis
- Product Name:Benzyl 6-Bromospiro[Indoline-3,4'-Piperidine]-1'-Carboxylate
- CAS Number:473737-32-5
- Molecular formula:C20H21BrN2O2
- Molecular Weight:401.3
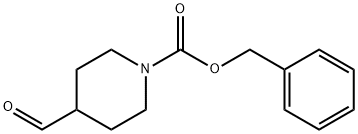
138163-08-3
193 suppliers
$6.00/1g
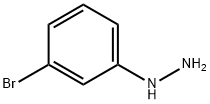
40887-80-7
22 suppliers
inquiry
![Benzyl 6-Bromospiro[Indoline-3,4'-Piperidine]-1'-Carboxylate](/CAS/GIF/473737-32-5.gif)
473737-32-5
32 suppliers
$226.00/25mg
Yield:473737-32-5 30%
Reaction Conditions:
Stage #1: N-(benzyloxycarbonyl)piperidine-4-carboxaldehyde;3-bromophenylhydrazinewith trifluoroacetic acid in dichloromethane at 0 - 40; for 18 h;
Stage #2: with sodium tetrahydroborate in dichloromethane at 0; for 0.5 h;
Steps:
1.1-1 Step 1-1, preparation of benzyl 6-bromo-1,2-dihydrospiro[indole-3,4'-piperidine]-1'- carboxylate:
Into a 250-mL round-bottom flask purged, were placed benzyl 4-formylpiperidine- 1-carboxylate (2.9 g, 12 mmol) and DCM (30 mL). The resulting solution was treated with (3- bromophenyl)hydrazine (2.0 g, 11 mmol) and then TFA (2.5 mL) at 0 C. The reaction mixture was stirred at 40 C for 18 h and cooled to rt. After cooling to 0 C, the reaction was treated with NaBH4 (0.82 g, 22 mmol) in several portions and stirred for 30 min. The resulting solution was diluted with water (20 mL) and added sat.-NaHCO3 to adjust the pH to 8~9. The solution was extracted with CH2Cl2 (2x). The combined organic layers were washed with brine, dried over anhydrous sodium sulfate and concentrated. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1:3). This resulted in the title compound (1.3 g, 30%) as yellow oil. LCMS (M+H)+ = 401.1.
References:
WO2021/133563,2021,A1 Location in patent:Paragraph 00305