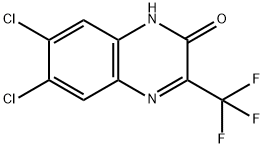
6,7-Dichloro-3-(trifluoromethyl)quinoxalin-2(1H)-one synthesis
- Product Name:6,7-Dichloro-3-(trifluoromethyl)quinoxalin-2(1H)-one
- CAS Number:477857-25-3
- Molecular formula:C9H3Cl2F3N2O
- Molecular Weight:283.03

477857-25-3
25 suppliers
$108.00/500mg
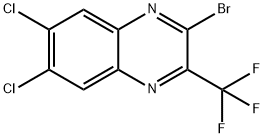
281209-27-6
0 suppliers
inquiry
Yield:281209-27-6 47%
Reaction Conditions:
with phosphorus tribromide at 140; for 16 h;
Steps:
60 2-Bromo-6,7-dichloro-3-trifluoromethylquinoxaline
6,7-Dichloro-3-trifluoromethyl-1H-quinoxalin-2-one (500 mg, 1.8 mmol) was dissolved in phosphorus tribromide (2.0 ml), and the solution was heated at 140° C. for 16 hours. The reaction mixture was cooled to room temperature, before it was poured out on ice (100 g) and extracted with dichloromethane. The organic phase was separated and dried with anhydrous sodium sulphate, then taken to dryness by rotary evaporation to leave a pale brown powder.Further purification using column chromatography and ethyl acetate:heptane (1:1) as eluent gave the title compound as a white powder. Yield: 295 mg (47%).1H-NMR (CDCl3): δ 8.25 (s, 1H); 8.35 (s, 1H).Anal. (calc. %; found %): C (31.25; 31.32); H (0.58; 0.61); N (8.10; 7.67).
References:
US6927214,2005,B1 Location in patent:Page/Page column 62