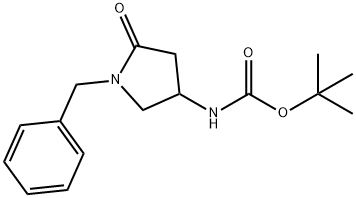
tert-Butyl (1-benzyl-5-oxopyrrolidin-3-yl)carbamate synthesis
- Product Name:tert-Butyl (1-benzyl-5-oxopyrrolidin-3-yl)carbamate
- CAS Number:478832-03-0
- Molecular formula:C16H22N2O3
- Molecular Weight:290.36
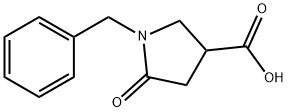
5733-86-8

75-65-0

478832-03-0
GENERAL STEPS: 1-benzyl-5-oxo-3-pyrrolidinecarboxylic acid (1.00 g, 4.56 mmol) was dissolved in tert-butanol (6 mL) followed by the addition of triethylamine (0.76 mL, 5.5 mmol). Next, a solution of diphenylphosphoryl azide (1.38 g, 5.02 mmol) in tert-butanol (4 mL) was slowly added dropwise to the reaction system. The resulting reaction mixture was heated to reflux for 2 hours. Upon completion of the reaction, the reaction solution was concentrated under reduced pressure and toluene was used as an azeotropic solvent to completely remove the tert-butanol. The residue was purified by silica gel column chromatography with the eluent being a mixed ethyl acetate/hexane solvent to give tert-butyl (1-benzyl-5-oxopyrrolidin-3-yl)carbamate (480 mg, 51% yield). The structure of the product was confirmed by 1H-NMR (DMSO-d6): δ 1.34 (9H, s), 2.23 (1H, dd, J = 5.7, 16.8 Hz), 2.61 (1H, dd, J = 8.6, 16.8 Hz), 3.01 (1H, dd, J = 5.7, 9.9 Hz), 3.43 (1H, dd, J = 8.6, 9.9 Hz), 4.03 (1H, m), 4.36 (2H, s), 7.30 (6H, m).

5733-86-8
154 suppliers
$39.00/1g

75-65-0
784 suppliers
$10.00/10ml

478832-03-0
25 suppliers
$7.00/250mg
Yield:478832-03-0 51%
Reaction Conditions:
with diphenylphosphoranyl azide;triethylamine for 2 h;Heating / reflux;
Steps:
207.b Example 207; Synthesis of N-(1-benzyl-5-oxopyrrolidin-3-yl)-1H-indazole-5-carboxamide; (b) Synthesis of tert-butyl 1-benzyl-5-oxo-3-pyrrolidinylcarbamate
In tert-butyl alcohol (6 ml) was dissolved 1-benzyl-5-oxo-3-pyrrolidinecarboxylic acid (1.00 g, 4.56 mmol), and triethylamine (0.76 ml, 5.5 mmol) was added thereto. Then, a solution of diphenylphosphoryl azide (1.38 g, 5.02 mmol) in tert-butyl alcohol (4 ml) was added thereto, and the resulting mixture was refluxed for 2 hours. After completion of the reaction, the reaction solution was concentrated and the tert-butyl alcohol was removed as an azeotrope with toluene as much as possible. The residue was purified by a silica gel chromatography (eluent: ethyl acetate/hexane) to obtain tert-butyl 1-benzyl-5-oxo-3-pyrrolidinylcarbamate (480 mg, 51%).1H-NMR (DMSO-d6) δ; 1.34 (9H, s), 2.23 (1H, dd, J=5.7, 16.8Hz), 2.61 (1H, dd, J=8.6, 16.8Hz), 3.01 (1H, dd, J=5.7, 9.9Hz), 3.43 (1H, dd, J=8.6, 9.9Hz), 4.03 (1H, m), 4.36 (2H, s), 7.30 (6H, m).
References:
EP1403255,2004,A1 Location in patent:Page 78-79
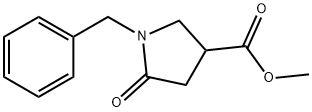
51535-00-3
136 suppliers
$8.00/1g

478832-03-0
25 suppliers
$7.00/250mg