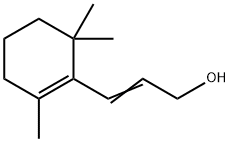
3-(2,6,6-Trimethyl-1-cyclohexene-1-yl)allyl alcohol synthesis
- Product Name:3-(2,6,6-Trimethyl-1-cyclohexene-1-yl)allyl alcohol
- CAS Number:4808-01-9
- Molecular formula:C12H20O
- Molecular Weight:180.29

4951-39-7
0 suppliers
inquiry

4808-01-9
0 suppliers
inquiry
Yield:4808-01-9 97.5%
Reaction Conditions:
with lithium aluminium tetrahydride in diethyl ether at 0; for 2 h;
Steps:
Preparation of Compound-3:
To a solution of LAH (8.2 g, 216 mmol) in anhydrous ether (168 mL) at 0°C was added a solution of Compound-2 (21 g, 108 mmol) in anhydrous ether. The reaction mixture was stirred at 0°C for 2 h. Then the reaction was quenched with a cold saturated NH4C1 solution and the resulting mixture was filtered through celite. The organic layer was separated from the filtrate, dried over anhydrous Na2504 and concentrated. The crude compound was purified by column chromatography on silica gel and eluted with 15-20% ethyl acetate/hexane to obtain Compound-3 (19 g, 97.5%). MS (m/z): [M - OH1, 163.3.
References:
WO2016/85939,2016,A2 Location in patent:Paragraph 00168
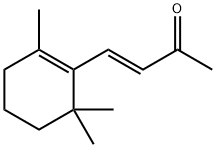
79-77-6
260 suppliers
$10.00/25g

4808-01-9
0 suppliers
inquiry

19834-52-7
1 suppliers
inquiry

4808-01-9
0 suppliers
inquiry
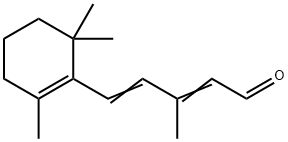
1209-68-3
2 suppliers
inquiry

4808-01-9
0 suppliers
inquiry