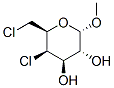
methyl 4,6-dichloro-4,6-dideoxy-alpha-galactopyranoside synthesis
- Product Name:methyl 4,6-dichloro-4,6-dideoxy-alpha-galactopyranoside
- CAS Number:4990-82-3
- Molecular formula:C7H12Cl2O4
- Molecular Weight:231.07
97-30-3
379 suppliers
$17.00/25g

4990-82-3
14 suppliers
inquiry
Yield:4990-82-3 60%
Reaction Conditions:
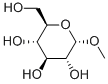
Stage #1: methyl-alpha-D-glucopyranosidewith pyridine;sulfuryl dichloride in chloroform at -78 - 50; for 42 h;
Stage #2: with sodium iodide in methanol;water at 20; for 0.166667 h;
Steps:
1
Sulfuryl chloride (16.7 mL, 206 mmol, 8.0 equiv) was added dropwise over 1 h to a solution of methyl a-D-glucopyranoside (5.0 g, 25.7 mmol) in pyridine (30 mL) and chloroform (30 mL) at -78 °C. The yellow suspension was stirred at -78 °C for 2 h and warmed to rt. The reaction mixture was heated to 50 °C and stirred for 40 h. After cooling to rt, the solution was diluted with methanol (15 mL) and water (15 mL) and subsequently neutralized by slow addition of solid sodium carbonate. A solution of sodium iodide (1.9 g, 12.7 mmol) in water (5 mL) and methanol (5 mL) was added to the reaction mixture, and the mixture was stirred an additional 10 min. The resulting mixture was filtered to remove insoluble matter and washed with chloroform (50 mL). The filtrate was separated into two layers and the aqueous layer was extracted with five 25-mL portions of chloroform. The combined organic layers were concentrated under reduced pressure. The residue was recrystallized from chloroform to provide methyl 4,6-dideoxy-4,6-dichloro-a-D- galactopyranoside (3.55 g, 60%) as a colorless solid. 1H NMR (CDC13, 500 MHz) δ = 4.85 (d, 1H, J = 3.8 Hz, H-l), 4.53 (d, 1H, J = 3.1 Hz, H-4), 4.14 (t, 1H, J = 6.5 Hz, H-5), 3.99 (dd, 1H, J = 9.8 Hz, 3.1 Hz, H-3), 3.85 (dd, 1H, J = 9.8 Hz, 3.8 Hz, H-2), 3.67 (d, 2H, J = 6.5 Hz, H-6), 3.48 (s, 3H, OCH3); 1JC NMR (CDC13, 100 MHz) δ = 99.4, 70.0, 69.8, 69.4, 62.6, 55.9, 42.8; HRMS (ESI) Calcd for C7Hi2Cl2Na04 [M+Na]+: 253.0010. Found: 253.0020.
References:
WO2014/165792,2014,A2 Location in patent:Paragraph 00715