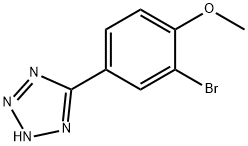
5-(3-BROMO-4-METHOXY-PHENYL)-2H-TETRAZOLE synthesis
- Product Name:5-(3-BROMO-4-METHOXY-PHENYL)-2H-TETRAZOLE
- CAS Number:191602-76-3
- Molecular formula:C8H7BrN4O
- Molecular Weight:255.07
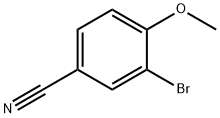
117572-79-9
207 suppliers
$9.00/1g

191602-76-3
25 suppliers
$329.00/1g
Yield:191602-76-3 96%
Reaction Conditions:
with sodium azide;ammonium cerium (IV) nitrate in N,N-dimethyl-formamide at 110; for 6 h;Inert atmosphere;Green chemistry;
Steps:
Experimental condition for 5-substituted 1-H tetrazoles (1b-16b)
General procedure: sodiumazide (1.5 mmol) was added to a magnetically stirred solution of nitrile 1a(1 mmol) in anhydrous DMF and the CAN (10 mmol %) was added. The reactionmixture was constantly stirred for another 6 h at 110 C under nitrogenatmosphere. After the completion of reaction as seen by TLC, the reactionmixture was brought to room temperature and the solvent was evaporatedunder vacuum. The crude thus obtained, was dissolved in ethyl acetate (20 mL)and solution was washed with acidified water (4 M HCl, 15 mL) twice.Separated organic layer was washed with brine solution dried overanhydrous Na2SO4, and solvent was removed under high vacuum to obtaintetrazole 1b as a white crystalline solid in 97% yield.
References:
Kumar, Satyanand;Dubey, Shristy;Saxena, Nisha;Awasthi, Satish Kumar [Tetrahedron Letters,2014,vol. 55,# 44,p. 6034 - 6038]