
5-(3-BROMO-PHENYL)-[1,3,4]THIADIAZOL-2-YLAMINE synthesis
- Product Name:5-(3-BROMO-PHENYL)-[1,3,4]THIADIAZOL-2-YLAMINE
- CAS Number:108656-65-1
- Molecular formula:C8H6BrN3S
- Molecular Weight:256.12
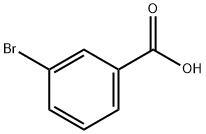
585-76-2
368 suppliers
$6.00/5g
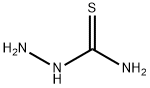
79-19-6
503 suppliers
$14.00/1g
![5-(3-BROMO-PHENYL)-[1,3,4]THIADIAZOL-2-YLAMINE](/StructureFile/ChemBookStructure4/GIF/CB8192945.gif)
108656-65-1
25 suppliers
$159.00/250mg
Yield:108656-65-1 89%
Reaction Conditions:
Stage #1: meta-bromobenzoic acid;thiosemicarbazidewith trichlorophosphate at 75; for 0.5 h;
Stage #2: with lithium hydroxide monohydrate for 4 h;Reflux;
Steps:
15 5.1.1.15. 5-(3-Bromophenyl)-1,3,4-thiadiazol-2-amine (74)
General procedure: 5.1.1
5-(4-Morpholinophenyl)-1,3,4-thiadiazol-2-amine (59)
A mixture of 4-morpholinobenzoic acid (5.18 g, 25 mmol) and N-aminothiourea (2.28 g, 25 mmol) in POCl3 (7 ml) was stirred vigorously at 75 °C for 0.5 h.
After addition of H2O (30 ml), the reaction mixture was heated under reflux for 4 h and basified to pH 8 by 50% NaOH solution.
The mixture was filtered and the filter cake was recrystallized from ethanol to yield 3.90 g of compound 59 as a yellow crystal. Yield: 59%; The synthetic procedures of compounds 60-81 were the same as that described above. 5.1.1.15
5-(3-Bromophenyl)-1,3,4-thiadiazol-2-amine (74)
Yield: 89%, mp: 222-224 °C (EtOH). ESI-MS m/z: 256.1 [M+H]+; 1H NMR (DMSO-d6) δ 7.43 (t, J = 7.8 Hz, 1H), 7.54 (s, 2H), 7.62-7.64 (m, 1H), 7.74 (d, J = 7.8 Hz, 1H), 7.93 (t, J = 1.8 Hz, 1H).
References:
Guan, Peng;Wang, Lei;Hou, Xuben;Wan, Yichao;Xu, Wenfang;Tang, Weiping;Fang, Hao [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 21,p. 5766 - 5775]
![Hydrazinecarbothioamide, 2-[(3-bromophenyl)methylene]-](/CAS/20180601/GIF/7420-35-1.gif)
7420-35-1
1 suppliers
inquiry
![5-(3-BROMO-PHENYL)-[1,3,4]THIADIAZOL-2-YLAMINE](/StructureFile/ChemBookStructure4/GIF/CB8192945.gif)
108656-65-1
25 suppliers
$159.00/250mg
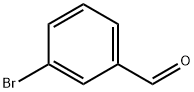
3132-99-8
506 suppliers
$5.00/10g

79-19-6
503 suppliers
$14.00/1g
![5-(3-BROMO-PHENYL)-[1,3,4]THIADIAZOL-2-YLAMINE](/StructureFile/ChemBookStructure4/GIF/CB8192945.gif)
108656-65-1
25 suppliers
$159.00/250mg

3132-99-8
506 suppliers
$5.00/10g
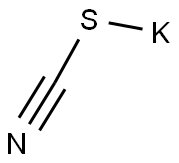
333-20-0
465 suppliers
$13.50/100G
![5-(3-BROMO-PHENYL)-[1,3,4]THIADIAZOL-2-YLAMINE](/StructureFile/ChemBookStructure4/GIF/CB8192945.gif)
108656-65-1
25 suppliers
$159.00/250mg

3132-99-8
506 suppliers
$5.00/10g
![5-(3-BROMO-PHENYL)-[1,3,4]THIADIAZOL-2-YLAMINE](/StructureFile/ChemBookStructure4/GIF/CB8192945.gif)
108656-65-1
25 suppliers
$159.00/250mg