
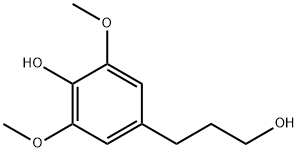
5-(3-Hydroxypropyl)-3-Methoxybenzene-1,2-diol synthesis
- Product Name:5-(3-Hydroxypropyl)-3-Methoxybenzene-1,2-diol
- CAS Number:132473-95-1
- Molecular formula:C10H14O4
- Molecular Weight:198.22
20736-25-8
79 suppliers
$100.00/100mg

132473-95-1
5 suppliers
inquiry

479547-23-4
0 suppliers
inquiry
Yield:479547-23-4 24% ,132473-95-1 17%
Reaction Conditions:
with zeolite beta (Zeolyst, CP814C) in water at 250; under 37503.8 Torr; for 6 h;Sealed tube;Inert atmosphere;Reagent/catalyst;
Steps:
IV.65 Example 65. O-demethylation of dihydrosinapylalcohol [JBO-946]
This experiment was performed according to a modified General procedure A. Dihydrosinapylalcohol (212 mg, 1 mmol) was used as the substrate, zeolite beta (Zeolyst, CP814C, Si02/Al203 = 38, H form) (100 mg) as acidic catalyst and H20 (2 mL) as the solvent. The reaction was performed at 250 °C for 6 h under 50 bar of N2 pressure. The zeolite was removed by filtration, using n-propanol, prior to freeze drying. 5-(3-Hydroxypropyl)benzene- 1 ,2,3-triol was obtained in 24% yield and the mono-demethylated intermediate in 17%. General procedure A (0233) A 4 mL glass vial was charged with a magnetic stirring bar, the substrate for the experiment, the acid or alkaline reagent and 2 mL of the appropriate solvent or solvent mixture. The vial was closed properly with the correct cap and septum and the septum was pierced with a syringe needle. This vial was brought to the 4620 Parr reactor and the reactor was closed properly. The reactor was flushed with the appropriate gas (3 x 10 bar) and then filled with this gas (with the reported pressure). The reactor was heated to the reaction temperature and this temperature was maintained for the reported reaction time (it takes approx. 60 min to reach 250 °C). After cooling down (from 250 °C to 170 °C in the air and from 170 °C to r.t. in an ice bath), the gas was released and the reactor was opened. (0234) After opening the reactor, the crude reaction mixture was brought to a roundbottomed flask and the vial was rinsed with H20 (3 mL). This aqueous reaction mixture was freezed by gently rotating the flask in liq. N2. Subsequently, vacuum was applied until all volatiles were removed. If necessary, this freeze drying step was repeated multiple times. The residue was redissolved in acetone, filtered over a silica plug and the filtrate was concentrated under reduced pressure by using a rotary evaporator. The residue was analysed with NMR and MS (APCI) or LC-MS.
References:
WO2019/25535,2019,A1 Location in patent:Page/Page column 23; 39