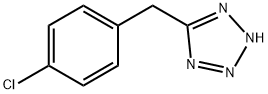
5-(4-CHLORO-BENZYL)-2H-TETRAZOLE synthesis
- Product Name:5-(4-CHLORO-BENZYL)-2H-TETRAZOLE
- CAS Number:14064-61-0
- Molecular formula:C8H7ClN4
- Molecular Weight:194.62
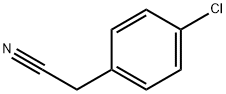
140-53-4
499 suppliers
$13.00/25g

14064-61-0
35 suppliers
$65.00/100mg
Yield:14064-61-0 96%
Reaction Conditions:
with sodium azide;ammonium chloride in N,N-dimethyl-formamide; for 24 h;Reflux;
Steps:
20 4.5. General procedure for the synthesis of compounds 1-24
General procedure: In a typical procedure, 5-aryl-1H-tetrazoles (1-24) were synthesized by adding aryl nitriles (1 eq.), sodium azide (1.2 eq.), and ammonium chloride (1 eq.) in solvent, the mixture was refluxed for 24 h. Progress of the reaction was monitored by thin layer chromatography. After completion of the reaction, 2.5 mL of 2M NaOH was added and the solution was stirred for half an hour. The reaction mixture was concentrated on reduced pressure, and dissolved in water. 3M HCl was added to the reaction mixture until precipitates formed. The precipitates were filtered and washed with distilled water. The yields of title compounds were found to be moderate to high.
References:
Fatima, Itrat;Zafar, Humaira;Khan, Khalid Mohammed;Saad, Syed Muhammad;Javaid, Sumaira;Perveen, Shahnaz;Choudhary, M. Iqbal [Bioorganic Chemistry,2018,vol. 79,p. 201 - 211]

20101-92-2
32 suppliers
$33.00/100mg

14064-61-0
35 suppliers
$65.00/100mg
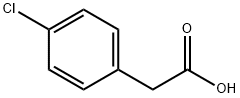
1878-66-6
453 suppliers
$6.00/25g

14064-61-0
35 suppliers
$65.00/100mg