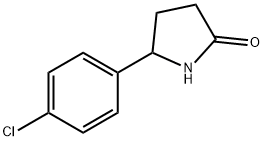
5-(4-Chlorophenyl)pyrrolidin-2-one synthesis
- Product Name:5-(4-Chlorophenyl)pyrrolidin-2-one
- CAS Number:279687-54-6
- Molecular formula:C10H10ClNO
- Molecular Weight:195.65
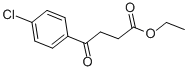
53503-49-4

279687-54-6
Step 2: Synthesis of intermediate M To a solution of ethyl 4-(4-chlorophenyl)-4-oxobutanoate L (81.9 mmol) in methanol (450 mL) was added ammonium acetate (63.1 g, 0.819 mol). The reaction mixture was stirred at 25 °C for 1 hour. Subsequently, sodium cyanoborohydride (5.14 g, 81.9 mmol) was added to the reaction system and stirring was continued under reflux conditions. After 18 hours of reaction, the reaction mixture was cooled to room temperature and subsequently concentrated under reduced pressure. The concentrated residue was dissolved in ethyl acetate (100 mL) and washed with water (3 x 50 mL). The organic phase was dried over anhydrous magnesium sulfate and concentrated again under reduced pressure. The residue was purified by silica gel column chromatography (eluent: ethyl acetate/methanol) to afford pure 5-(4-chlorophenyl)pyrrolidin-2-one M. For example, the target product obtained by isolation was obtained in 60% yield. 1H NMR (CDCl3) δ (ppm): 7.34 (2H, d), 7.25 (2H, d), 6.15 (1H, bs), 4.74 (2H, t), 2.62-2.50 (1H, m), 2.50-2.35 (2H, m), 1.98-1.89 (1H, m). LC/MS (M + H)+: 196.

53503-49-4
26 suppliers
$303.00/1g

279687-54-6
23 suppliers
$141.00/100mg
Yield: 60%
Reaction Conditions:
Stage #1:ethyl 4-(4-chlorophenyl)-4-oxobutanoate with ammonium acetate in methanol at 25; for 1 h;
Stage #2: with sodium cyanoborohydride in methanol for 18 h;Reflux;
Steps:
2.2 Step 2: Intermediate M
Step 2: Intermediate M [0065] To phenyl 4-oxobutanoate ester L (81.9 mmol) in MeOH (450 mL) was added ammonium acetate (63.1 g, 0.819 mol). The reaction mixture was stirred at 25 °C for 1 hour. Sodium cyanoborohydride (5.14 g, 81.9 mmol) was added to the reaction and stirred at reflux. After 18 hours the reaction was cooled and then concentrated under reduced pressure. The residue was dissolved in ethyl acetate (100 mL) and washed with water (3 × 50mL). The organic phase was dried (MgSO4) and concentrated down under reduced pressure. The residue was purified by chromatography (silica, EtOAc/MeOH) to afford pure pyrrolidin-2-one M. For example, 5-(4-chlorophenyl)pyrrolidin-2-one was isolated in 60% yield. 1H NMR δ (ppm)(CHCl3-d): 7.34 (2 H, d), 7.25 (2H, d), 6.15 (1H, bs), 4.74 (2H, t), 2.62-2.50 (1H, m), 2.50-2.35 (2H, m), 1.98-1.89 (1H, m). LC/MS (M+H)+ 196
References:
ARES TRADING S.A.;Whittaker, Ben;Steele, Chris;Hardick, David;Dale, Mitchell;Pomel, Vincent;Quattropani, Anna;Beher, Dirk EP2687528, 2014, A1 Location in patent:Paragraph 0065
3984-34-7
145 suppliers
$7.00/1g

279687-54-6
23 suppliers
$141.00/100mg
4619-18-5
74 suppliers
$17.00/250mg

279687-54-6
23 suppliers
$141.00/100mg
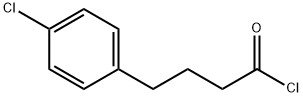
54654-08-9
1 suppliers
inquiry

279687-54-6
23 suppliers
$141.00/100mg

1749-03-7
4 suppliers
inquiry

279687-54-6
23 suppliers
$141.00/100mg