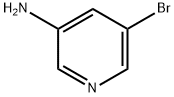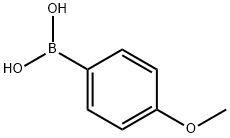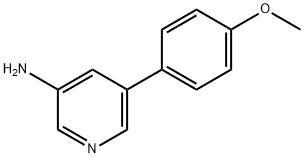
5-(4-Methoxyphenyl)pyridin-3-aMine synthesis
- Product Name:5-(4-Methoxyphenyl)pyridin-3-aMine
- CAS Number:1225522-97-3
- Molecular formula:C12H12N2O
- Molecular Weight:200.24
Yield:1225522-97-3 44%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in water;N,N-dimethyl-formamide at 120; for 0.166667 h;Inert atmosphere;Microwave irradiation;
Steps:
III-1.1
A mixture of 5-bromopyridin-3-amine (300 mg, 1.74 mmol), 4- methoxyphenylboronic acid (395 mg, 2.60 mmol), K2C03 (240 mg, 5.10 mmol) and Pd(PPh3)4 (197 mg, 0.17 mmol) in DMF/H20 (5 ml/1 ml) was purged with N2 for 20 min. Then the mixtuer was stirred at 120°C under microwave irridation for 10 min. After cooled to room temperature, the solvent was removed in vacuum. The residue was diluted with EtOAc (30 mL). The mixture was washed with water, brine and dried over Na2S04. The solution was evaported to dryness and purified by silica gel column (DCM/MeOH, 1/0-40/1) to afford 264 mg (yield: 44%) of 5-(4-methoxyphenyl)pyridin-3-amine as white solid. MS: m z 201.1 (M+H+).
References:
WO2013/126608,2013,A1 Location in patent:Paragraph 00279