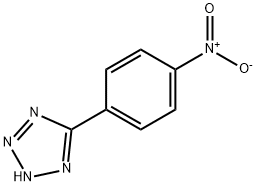
5-(4-NITRO-PHENYL)-2H-TETRAZOLE synthesis
- Product Name:5-(4-NITRO-PHENYL)-2H-TETRAZOLE
- CAS Number:16687-60-8
- Molecular formula:C7H5N5O2
- Molecular Weight:191.15
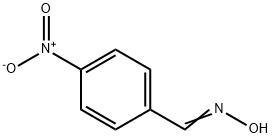
1129-37-9

16687-60-8
The general procedure for the synthesis of 5-(4-nitrophenyl)-1H-tetrazole from 4-nitrophenyl aldoxime was as follows: InCl3 (3 mol%) was added to a stirred solution of DMF (5 mL) containing appropriate amounts of aldoxime (1 mmol) and NaN3 (1.5 mmol). The reaction mixture was heated to 120 °C. After completion of the reaction (monitored by TLC), the mixture was cooled to room temperature. H2O (5 mL), 2M HCl aqueous solution (10 mL) and EtOAc (10 mL) were added sequentially with vigorous stirring for 15 minutes. The organic layer was separated and the aqueous layer was extracted with EtOAc (3 x 15 mL). The organic extracts were combined, washed with H2O, dried over anhydrous Na2SO4 and filtered. The solvent was evaporated under reduced pressure and the crude product was purified by silica gel column chromatography [eluent: EtOAc-hexane (9:1)].

1129-37-9
100 suppliers
$18.00/1g

16687-60-8
93 suppliers
$15.00/100mg
Yield: 85%
Reaction Conditions:
with sodium azide;copper(II) ferrite in N,N-dimethyl-formamide at 120; for 12 h;Sealed tube;
Steps:
General Procedure for the Synthesis of 5-substituted 1H-tetrazoles
General procedure: A mixture of oxime (1 mmol), NaN3 (1.5 mmol), catalyst(30 mol %) and DMF (3 mL) was taken in a sealed tube and stirred at 120 °C for 12 h. After completion of the reaction,the catalyst was separated from the reaction mixture with an external magnet kept at RT for 5 min. Then H2O (5 mL) and3N HCl (5 mL) were added, and an extraction with ethylacetate was performed. Resultant organic layer was washedwith water, dried over sodium sulfate, and the solvent was removed by distillation. The crude material was purified by column chromatography on silica gel (hexane/AcOEt 1:1)and afforded the pure tetrazole derivatives.
References:
Akula, Ravi Kumar;Adimulam, Chandra S.;Gangaram, Sathaiah;Kengiri, Raju;Banda, Narsaiah;Pamulaparthy, Shanthan R. [Letters in Organic Chemistry,2014,vol. 11,# 6,p. 440 - 445]

1129-37-9
100 suppliers
$18.00/1g

16687-60-8
93 suppliers
$15.00/100mg
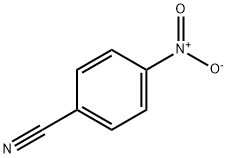
619-72-7
380 suppliers
$11.00/1g

16687-60-8
93 suppliers
$15.00/100mg
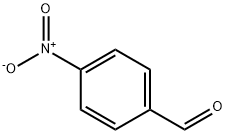
555-16-8
574 suppliers
$5.00/5g

16687-60-8
93 suppliers
$15.00/100mg
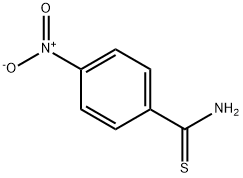
26060-30-0
37 suppliers
$25.00/1g

16687-60-8
93 suppliers
$15.00/100mg