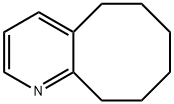
5,6,7,8,9,10-hexahydrocycloocta[b]pyridine synthesis
- Product Name:5,6,7,8,9,10-hexahydrocycloocta[b]pyridine
- CAS Number:28712-60-9
- Molecular formula:C11H15N
- Molecular Weight:161.24
Yield:28712-60-9 78 %Chromat.
Reaction Conditions:
with C19H15AuClN2PS;silver trifluoromethanesulfonate in ethanol at 120; for 5 h;
Steps:
General procedures for the synthesis of pyridine derivativecatalyzed by the Au(I)-complex
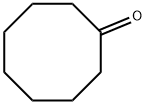
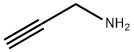
General procedure: In this part, the developed Au(I)-complexes of Au-L1 and Au-L2were applied as the pre-catalysts for the one-pot reaction of 1,3-diphenylacetone and propargylamine for the efficient synthesis ofpyridine derivatives (Table 3).Without the involvement of any auxiliary additive, under theselected reaction conditions, over Au-L1 (2.5 mol%) the conversionsof 1,3-diphenylacetone were steadily increased as the reaction tem-perature increased from 70 to 140C along with 100% selectivityto 2-benzyl-3-phenylpyridine (Entries 1-4). And 90% yield of 2-benzyl-3-phenylpyridine was obtained at 120C after screeningethanol as the optimal solvent (Entry 4, 95%). In contrast, underthe same reaction conditions, Au-L2 exhibited much lower activ-ity towards the reaction with the product yield of 53% (Entry10). The involvement of AgOTf (silver trifluoromethanesulfonate)in Au-L1 or Au-L2 as a scavenger of Cl-ligand for the benefi-cial formation of P-Au+species [39,40] showed negligible effecton their catalytic performance (Entries 12 and 13). While N-methylimidazole was added additionally in Au-L2, the yield of2-benzyl-3-phenylpyridine was improved up to 75%, but still muchlower than that obtained over Au-L1 (Entry 11 vs 3).As verified in Fig. 2 the coordination abilities of the phosphinefragments in L1 and L2 are very similar (L1,1J31P-77Se= 758 Hz;L2,1J31P-77Se= 761 Hz). Hence, the different catalytic performanceof Au-L1 and Au-L2 towards above reaction was not due to thechange of the coordinating ability of the involved phosphines in L1and L2. Reasonably, the presence of N-containing moiety in themode of the intramolecular incorporation in Au-L1 contributedto the high efficiency of this sequential condensation/annulationreaction (Entry 3). Au-L1 was able to act as a unique bifunctionalcatalyst in combination of the phosphine-ligated Au-complex (asthe transition metal catalyst) and the Lewis base organocatalystderived from imidazolyl group (Scheme 4). Due to the rigid andbulky backbone of thiophenylimidazolyl moiety, the incorporatedimidazolyl, which was located remotely to the phosphine frag-ment without competitive N-hemilabile ligation to Au-center, justplayed the role of Lewis base to effectively catalyze the con-densation of 1,3-diphenylacetone with propargylamine for theformation of the imino intermediate with enamino tautomer (A).Since the exclusive alkynophilicity of the Au-complex catalyst[16,17], carbon-carbon triple bond was activated by P-Au+speciesthrough the -coordination, and then attached by the carbon ofthe enamino moiety through regioselective 6-endo-dig intramolec-ular nucleophilic addition to give dehydropyridine intermediate(B). The latter undergoes auto-oxidation reaction to afford thepyridine derivatives. In contrast, Au-L2 without the incorporatedLewis base exhibited lower activity with the product yield of 53%(Entry 10). Although, the sequential reaction efficiency over themechanical mixtures of Au-L2 and N-methylimidazole could beimproved to some extent (75% yield, Entry 11), it was still lowerthan that over Au-L1. These results further confirmed that theintramolecular combination of the Au-complex unit with the imi-dazolyl ring by the stable chemical bonds in Au-L1 was not theresult of the simple mechanical mixture of the phosphine-ligatedAu-complex of Au-L2 and the Lewis base of N-methylimidazole.In the mechanical mixtures of Au-L2 and N-methylimidazole, theauxiliary N-methylimidazole as an N-containing donor with thefree mobility unavoidably competed with the alkyne substrate tocoordinate to Au-center, leading to the depressed condensation ofketone and amine over the basic imidazole catalyst and the subse-quent activation of alkyne for annulation over Au-L2 catalyst.The generality of Au-L1 as the bi-functional catalyst for the syn-theses of the pyridine derivatives was examined on the differentscope of carbonyl compounds. As shown in Table 4, under theselected reaction conditions, Au-L1 universally exhibited accept-able catalytic activities towards the sequential transformation.The steric and electronic effects of the applied ketones had theobvious influence on the reaction rate. The cycloalkanones withexposed C O groups gave rise to the formation of 2,3-fusedpyridines with the good isolated yields (Entries 1-4, 53-89%),in which cyclopentanone with the least steric hindrance corre-sponded to the best yield of cyclopentylpyridine (Entry 3, 89%).The reaction of the less activated aryl/(heteroaryl)-methyl ketoneswith propargylamine led to the formation of 2-aryl(heteroaryl)-substituted pyridines with the moderate yields (Entries 5-9), whichrepresented an alternative to the Suzuki cross-coupling protocol[41]. The involved substitutes with intensive electron withdraw-ing effect (such as -Br/-F/pyridinyl) dramatically slowed down thereaction rate (Entries 6-9). Under the prolonged reaction time(10 h), the aliphatic aldehydes in general gave 3-alkyl-substitutedpyridines with 92% yield of 3-phenylpyridine and 48% yield of 3-benzylpyridine (Entries 10 and 11)..
References:
Yang, Da;Liu, Huan;Wang, Dong-Liang;Lu, Yong;Zhao, Xiao-Li;Liu, Ye [Journal of Molecular Catalysis A: Chemical,2016,vol. 424,p. 323 - 330]

63665-46-3
0 suppliers
inquiry
![5,6,7,8,9,10-hexahydrocycloocta[b]pyridine](/CAS/GIF/28712-60-9.gif)
28712-60-9
4 suppliers
inquiry