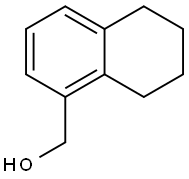
(5,6,7,8-Tetrahydronaphthalen-1-yl)Methanol synthesis
- Product Name:(5,6,7,8-Tetrahydronaphthalen-1-yl)Methanol
- CAS Number:41790-30-1
- Molecular formula:C11H14O
- Molecular Weight:162.23
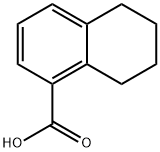
4242-18-6

41790-30-1
General procedure for the synthesis of 1-(hydroxymethyl)-5,6,7,8-tetrahydronaphthalene from 5,6,7,8-tetrahydro-1-naphthalenecarboxylic acid: 5,6,7,8-tetrahydro-1-naphthalenecarboxylic acid (2.0 g, 11 mmol) was dissolved in tetrahydrofuran (THF, 30 mL), and the borane-THF solution was slowly added at 0°C (23 mL, 0.98 M THF solution. 23 mmol). The reaction mixture was stirred at room temperature for 16 hours. Upon completion of the reaction, the reaction was quenched by careful addition of water at 0 °C, followed by extraction with ethyl acetate. The organic layer was washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography with gradient elution from hexane to hexane/ethyl acetate (3:1, v/v) as eluent to give 1-(hydroxymethyl)-5,6,7,8-tetrahydronaphthalene (1.9 g, colorless oil, quantitative yield).

4242-18-6
207 suppliers
$6.00/100mg

41790-30-1
57 suppliers
$132.00/250mg
Yield:41790-30-1 100%
Reaction Conditions:
with borane-THF in tetrahydrofuran at 0 - 20; for 16 h;
Steps:
1 synthesis of (5,6,7,8-tetrahydronaphthalene 1-yl)methanol
5,6,7,8-tetrahydronaphthalene 1-carboxylic acid (2.0 g, 11mmol) was dissolved in the tetrahydrofuran (following, THF) (30 mL), borane.THF (23 mL, a 0.98M THF solution, 23mmol) were added at 0 degree C, and it agitated at the room temperature for 16 hours. Water was added to the reaction solution at 0 degree C, and ethyl acetate extracted. The saturated sodium chloride solution washed the organic layer, and it condensed after drying with anhydrous sodium sulfate, and obtained the rough product. silica gel column chromatography (n-hexane -> n-hexane/ethyl acetate =3/1) refined the obtained rough product, and it obtained the title compound (1.9 g and colourless oil, quantitive)
References:
JP2016/222570,2016,A Location in patent:Paragraph 0034
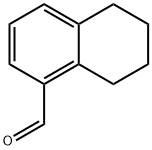
41828-13-1
50 suppliers
$88.00/100mg

41790-30-1
57 suppliers
$132.00/250mg
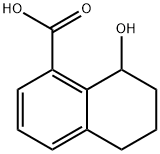
74145-11-2
1 suppliers
inquiry

41790-30-1
57 suppliers
$132.00/250mg
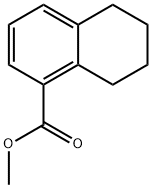
66193-59-7
29 suppliers
inquiry

41790-30-1
57 suppliers
$132.00/250mg
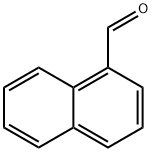
66-77-3
388 suppliers
$7.00/10g

41790-30-1
57 suppliers
$132.00/250mg