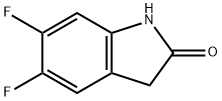
5,6-Difluoro-2-oxoindole synthesis
- Product Name:5,6-Difluoro-2-oxoindole
- CAS Number:71294-07-0
- Molecular formula:C8H5F2NO
- Molecular Weight:169.13
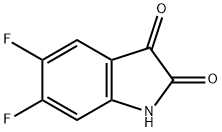
774-47-0

71294-07-0
General procedure for the synthesis of 5,6-difluoroindolin-2,3-dione from 5,6-difluoroindolin-2-one: TiCl4 (0.7 mL, 6 mmol) was added drop-wise to a stirring suspension of Zn powder (0.78 g, 12 mmol) in freshly distilled anhydrous THF (15 mL) at room temperature and under dry N2 atmosphere. After addition, the mixture was refluxed for 2 hours. The resulting suspension of low-valent titanium reagent was cooled to room temperature. Subsequently, a solution of 5,6-difluoroindoline-2,3-dione (2 mmol) in THF (10 mL) was slowly added. The reaction mixture was stirred at room temperature for about 5 min under N2 protection. After confirming the completion of the reaction by thin layer chromatography (TLC) analysis, the reaction was quenched with 3% HCl (15 mL) and extracted with CHCl3 (3 x 50 mL). The organic phases were combined, washed with water (3 x 50 mL) and dried over anhydrous Na2SO4. After concentration of the solvent under reduced pressure, the crude product was purified by column chromatography (petroleum ether/ethyl acetate=5:1) to afford the target compound 5,6-difluoroindol-2-one.

774-47-0
73 suppliers
$8.00/250mg

71294-07-0
110 suppliers
$45.00/50mg
Yield: 89%
Reaction Conditions:
with titanium tetrachloride;zinc in tetrahydrofuran at 20; for 0.0833333 h;Inert atmosphere;
Steps:
General procedure for the synthesis of compounds 2 and 4
General procedure: TiCl4 (0.7 mL, 6 mmol)was added to a stirred suspension of Zn powder (0.78 g, 12 mmol) in freshlydistilled anhydrous THF (15 mL) at room temperature (rt) under a dry N2atmosphere. After completion of the addition, the mixture was refluxed for 2 h.The suspension of the low-valent titanium reagent thus-formed was cooled tort. A solution of isatin or its derivatives 1 or 3 (2 mmol) in THF (10 mL) wasadded dropwise. The mixture was stirred at room temperature for about 5 minunder N2. After this period, the thin layer chromatography (TLC) analysis of themixture showed the reaction completed. The reaction mixture was quenchedwith 3% HCl (15 mL) and extracted with CHCl3 (3 50 mL). The combinedextracts were washed with water (3 50 mL) and dried over anhydrousNa2SO4. After evaporation of the solvent under reduced pressure, the crudeproduct was purified by column chromatography (petroleum ether/ethylacetate = 5:1) to give the pure products 2 or 4.
References:
Lin, Wei;Hu, Ming-Hua;Feng, Xian;Fu, Lei;Cao, Cheng-Pao;Huang, Zhi-Bin;Shi, Da-Qing [Tetrahedron Letters,2014,vol. 55,# 14,p. 2238 - 2242]
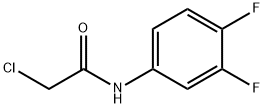
76778-13-7
36 suppliers
$24.00/250 mg

71294-07-0
110 suppliers
$45.00/50mg
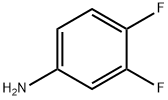
3863-11-4
344 suppliers
$10.00/25 g

71294-07-0
110 suppliers
$45.00/50mg