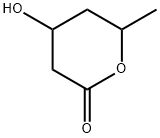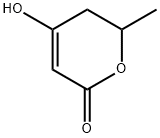
5,6-DIHYDRO-4-HYDROXY-6-METHYL-2H-PYRAN-2-ONE synthesis
- Product Name:5,6-DIHYDRO-4-HYDROXY-6-METHYL-2H-PYRAN-2-ONE
- CAS Number:33177-29-6
- Molecular formula:C6H8O3
- Molecular Weight:128.13
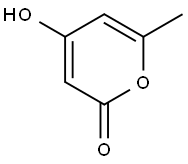
675-10-5
245 suppliers
$9.00/1g

33177-29-6
22 suppliers
$203.00/1g
Yield:33177-29-6 92%
Reaction Conditions:
with hydrogen;5 % Pd/Nb2O5 in butan-1-ol at 69.84; under 5171.62 Torr; for 5 h;
Steps:
1
The reactions were performed in a 50 mL batch reactor (Parr Instruments, Moline, Ill.). 5wt % Pd/Nb2O5 was used as the catalyst, with 1.8wt % HMP as the reactant in all cases. The mass ratio of catalyst to HMP was 1:1.7 in all cases. It was found that increased temperature yielded increased product degradation. It was also found that higher H2 pressures caused further hydrogenation of DHHMP to 4-HMTHP and subsequently to δ-hexalactone (HL). Additionally, the solvent has an effect on product selectivity. The highest selectivity to DHHMP (92%, see Table 1) was attained when 1-butanol was employed as the solvent. The complete hydrogenation reaction from HMP (1) to 4-HMTHP (3) is depicted in Reaction Scheme 2. Table 1 presents the results for the partial hydrogenation reaction of HMP (1) to DHHMP (2) over 5 wt % Pd/Nb2O5 at various temperatures, hydrogen partial pressures, and using different solvents.
References:
US2012/116119,2012,A1 Location in patent:Page/Page column 5
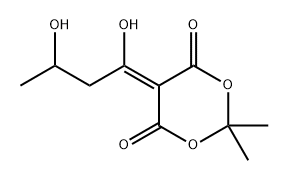
84257-13-6
0 suppliers
inquiry

33177-29-6
22 suppliers
$203.00/1g
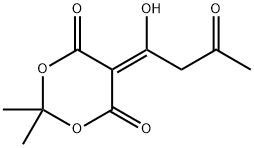
84257-12-5
0 suppliers
inquiry

33177-29-6
22 suppliers
$203.00/1g
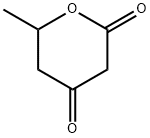
85825-79-2
9 suppliers
inquiry

33177-29-6
22 suppliers
$203.00/1g