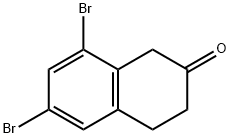
5,7-dibromo-2-tetralone synthesis
- Product Name:5,7-dibromo-2-tetralone
- CAS Number:144066-44-4
- Molecular formula:C10H8Br2O
- Molecular Weight:303.98
Yield:144066-44-4 10% ,1131456-53-5 6%
Reaction Conditions:
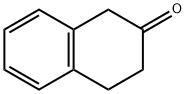
Stage #1: 1,2,3,4-tetrahydronaphthalen-2-one;aluminum (III) chloride at 0 - 90; for 0.666667 h;
Stage #2: with bromine at 85; for 1 h;
Steps:
III
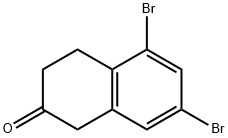
,7-Dibromo-2-tetralone and 6,8-dibromo-2-tetralone. Into a round bottom flask kept at 0° C., AlCl3 (2.26 g, 16.9 mmol) was added and the reaction system was put under nitrogen. 1 mL of β-tetralone (1.08 g, 7.37 mmol) were added during a 10-minute period, at which point, the reaction mixture was heated in an oil bath at 90° C. for about 30 minutes before adding 0.45 mL of Br2 (1.41 g, 8.82 mmol). The reaction mixture was stirred at 85° C. for an hour before 15 mL of ice-water and 8 mL of NaHCO3 were added. The product was extracted twice from the aqueous phase with Et2O and, the resultant organic phase was washed once with NaHCO3, once with brine and dried under Na2SO4. The crude reaction mixture was purified by flash chromatography (10% EtOAc/90% hex) which afforded 2 major compounds. 0.110 g (0.362 mmol) of 6,8-dibromo-2-tetralone (less polar compound) was obtained in a 6% yield.1H NMR (300 MHz, CDCl3, δ): 7.34 (s, ArH, 2H), 3.66 (s, CH2, 2H), 3.24 (t, J=6.8, CH2, 2H), 2.60 (t, J=6.9, CH2, 2H).13C NMR (75 MHz, CDCl3): δ 207.8, 137.9, 135.1, 131.93, 131.90, 123.2, 122.9, 44.6, 37.9, 29.4.Dept 135 NMR (75 MHz, CDCl3): δ 131.99 (CH), 131.95 (CH), 44.7 (CH2), 38.0 (CH2), 29.5 (CH2).The more polar compound was recrystallized (MeOH-hex) to yield 0.2602 g (0.856 mmol) of 5,7-dibromo-2-tetralone in a 10% yield.1H NMR (300 MHz, CDCl3, δ): 7.64 (d, J=1.8, ArH, 1H), 7.34 (d, J=1.8, ArH, 1H), 3.58 (s, CH2, 2H), 3.06 (t, J=6.5, CH2, 2H), 2.57 (t, J=6.7, CH2, 2H).13C NMR (75 MHz, CDCl3): δ 207.9, 140.1, 133.3, 133.2, 130.4, 124.7, 120.6, 44.1, 38.1, 29.0.Dept 135 NMR (75 MHz, CDCl3): δ 133.2 (CH), 130.1 (CH), 44.1 (CH2), 38.1 (CH2), 29.0 (CH2).
References:
US2009/76076,2009,A1 Location in patent:Page/Page column 21