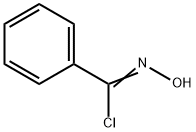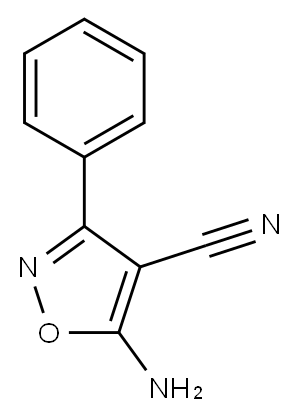
5-amino-3-phenyl-1,2-oxazole-4-carbonitrile synthesis
- Product Name:5-amino-3-phenyl-1,2-oxazole-4-carbonitrile
- CAS Number:14246-77-6
- Molecular formula:C10H7N3O
- Molecular Weight:185.1821
Yield:14246-77-6 65.2%
Reaction Conditions:
with sodium ethanolate in ethanol at 0 - 20; for 4 h;
Steps:
1.4. Synthetic procedures for 5-amino-3-phenylisoxazole-4-carbonitrile (4)
A malononitrile (3 g, 45.45 mmol) was added partwise in a solution of sodium ethoxide (6.18 g, 90.90 mmol) in ethanol (30 mL) over 20 min in cooling at 0°C, and after addition the mixture was stirred for another 30 min. Then a solution of (E/Z)-N-hydroxybenzimidoyl chloride (7 g, 45.45 mmol) in cold ethanol was added dropwise to the above mixture at cooling condition after the addition is over the reaction mixture stirred at room temperature. Reaction monitored using TLC, after the completion of the reaction, the mixture was carefully added to crushed ice, yielding an orange-yellow solid suspension. This suspension was stirred for 5 min, after the formation of a precipitate, the mixture was filtered and washed with hexane and dried to yield an off white solid of 5-amino-3-phenylisoxazole-4-carbonitrile (4); 65.2% yield. m.p.:146-148°C. 1H NMR (500 MHz, DMSO-d6) δ 8.58 (s, 2H), 7.93 - 7.63 (m, 2H), 7.67 - 7.13 (m, 3H). 13C NMR (126 MHz, DMSO-d6) δ 174.22, 161.58, 131.38, 129.64, 127.68, 127.26, 114.14, 63.64. HRMS -QTOF MS/MS: m/z [M + H]+ calcd for C10H8N3O: 186.0667; found: 186.0659.
References:
Bansod, Sapana;Gaikwad, Nikhil Baliram;Garise, Ramana;Godugu, Chandraiah;Mara, Alekhya;Srinivas, Nanduri;Yaddanapudi, Venkata Madhavi [Bioorganic and Medicinal Chemistry Letters,2021,vol. 49,art. no. 128294] Location in patent:supporting information

60776-91-2
0 suppliers
inquiry

14246-77-6
4 suppliers
inquiry

100-52-7
972 suppliers
$20.37/250g

14246-77-6
4 suppliers
inquiry

932-90-1
152 suppliers
$17.67/1gm:

14246-77-6
4 suppliers
inquiry
![2-[methoxy(phenyl)methylene]malononitrile](/CAS/20180629/GIF/42754-56-3.gif)
42754-56-3
3 suppliers
inquiry

14246-77-6
4 suppliers
inquiry