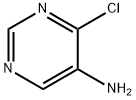
5-AMINO-4-CHLOROPYRIMIDINE synthesis
- Product Name:5-AMINO-4-CHLOROPYRIMIDINE
- CAS Number:54660-78-5
- Molecular formula:C4H4ClN3
- Molecular Weight:129.55
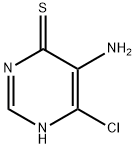
54851-35-3

54660-78-5
General procedure for the synthesis of 5-amino-4-chloropyrimidine from 5-amino-6-chloropyrimidine-4-thiol: The concentrated product of Example 18 (4.3 g, 26 mmol) was dissolved in EtOH (40 mL). Subsequently, NH4OH (4 mL) was added to this solution. Under stirring, an excess of Raney Nickel was added in batches. the reaction mixture was stirred overnight at room temperature and then heated at 80 °C for 2 h. The reaction was carried out at room temperature. Upon completion of the reaction, the mixture was filtered through a diatomaceous earth pad and the filtrate was concentrated. The crude product was purified by silica fast chromatography using EtOAc/hexane as eluent to give 1.6 g (47% yield) of 5-amino-4-chloropyrimidine as a yellow solid.

54851-35-3
34 suppliers
inquiry

54660-78-5
156 suppliers
$13.00/100mg
Yield: 47%
Reaction Conditions:
with ammonia;water;nickel in ethanol at 20 - 80;
Steps:
19
To the product of Example 18 (4.3 g, 26 mmol) dissolved in conc. NH4OH (4 mL) was added EtOH (40 mL). To this solution, Raney Nickel (excess) was added in portions. The reaction was stirred at room temperature overnight and then heated at 80° C. for 2 hrs. The mixture was filtered through Celite and the filtrate concentrated. The crude product was purified by flash chromatography on silica using EtOAc/hexanes to afford 1.6 g (47%) of the title compound as a yellow solid.
References:
Elan Pharmaceutical Inc. US2006/13799, 2006, A1 Location in patent:Page/Page column 59

54851-35-3
34 suppliers
inquiry

54660-78-5
156 suppliers
$13.00/100mg