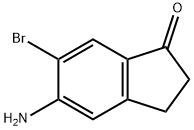
5-amino-6-bromo-2,3-dihydro-1H-inden-1-one synthesis
- Product Name:5-amino-6-bromo-2,3-dihydro-1H-inden-1-one
- CAS Number:158205-19-7
- Molecular formula:C9H8BrNO
- Molecular Weight:226.07
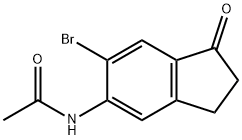
158205-18-6

158205-19-7
General procedure for the synthesis of 5-amino-6-bromo-2,3-dihydro-1H-inden-1-one from N-(6-bromo-1-oxo-2,3-dihydro-1H-inden-5-yl)acetamide: N-(6-bromo-1-oxo-2,3-dihydro-1H-inden-5-yl)acetamide (13.1 g, 48.9 mmol) was reacted with 6 M aqueous hydrochloric acid solution (260 mL. 1.560 mol) were mixed and the reaction was stirred at 100 °C for 1 hour. After completion of the reaction, the reaction solution was cooled to 0 °C and the pH was adjusted to 8 with 10 M aqueous sodium hydroxide.The precipitated solid was collected by filtration, washed with water and dried in vacuum to afford the light brown powdery product 5-amino-6-bromo-2,3-dihydro-1H-inden-1-one (10.8 g, 98% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.86 (s, 1H), 6.74 (s, 1H), 4.68 (s, 2H), 2.98 (t, J = 6.0 Hz, 2H), 2.64 (t, J = 6.0 Hz, 2H).

158205-18-6
18 suppliers
inquiry

158205-19-7
40 suppliers
$47.00/100mg
Yield:158205-19-7 98%
Reaction Conditions:
with hydrogenchloride in water at 100; for 1 h;Inert atmosphere;
Steps:
499.D Step D: 5-Amino-6-bromo-indan-1-one
A mixture of N-(6-bromo-1-oxo-indan-5-yl)acetamide (13.1 g, 48.9 mmol) and 6 M aqueous hydrochloric acid (260 ml, 1.560 mol) was stirred at 100 oC for 1 h under nitrogen. The solution was cooled to 0 oC and adjusted to pH = 8 with 10 M aqueous sodium hydroxide solution. The precipitate formed was collected, washed with water, and dried under vacuum to afford 5-amino- 6-bromo-indan-1-one (10.8 g, 98%) as a light brown powder. 1H NMR (400 MHz, CDCl3) δ 7.86 (s, 1H), 6.74 (s, 1H), 4.68 (s, 2H), 2.98 (t, J = 6.0 Hz, 2H), 2.64 (t, J = 6.0 Hz, 2H).
References:
F. HOFFMANN-LA ROCHE AG;GENENTECH, INC.;BRYAN, Marian, C.;GOBBI, Alberto;KIEFER, James, Richard, Jr.;KOLESNIKOV, Aleksandr;OLIVERO, Alan, G.;DROBNICK, Joy;LIANG, Jun;RAJAPAKSA, Naomi WO2017/108723, 2017, A2 Location in patent:Page/Page column 749
24425-40-9
206 suppliers
$55.00/10 g

158205-19-7
40 suppliers
$47.00/100mg
157701-33-2
31 suppliers
$198.00/100 mg

158205-19-7
40 suppliers
$47.00/100mg
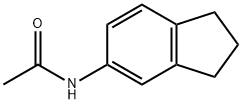
59856-06-3
42 suppliers
$60.00/1 g

158205-19-7
40 suppliers
$47.00/100mg