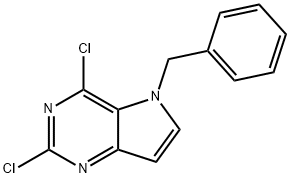
5-benzyl-2,4-dichloro-5H-pyrrolo[3,2-d]pyriMidine synthesis
- Product Name:5-benzyl-2,4-dichloro-5H-pyrrolo[3,2-d]pyriMidine
- CAS Number:129872-83-9
- Molecular formula:C13H9Cl2N3
- Molecular Weight:278.14
![4-chloro-5-benzyl-5H-Pyrrolo[3,2-d]pyrimidin-2-amine](/CAS/20180703/GIF/914496-94-9.gif)
914496-94-9
2 suppliers
inquiry
![5-benzyl-2,4-dichloro-5H-pyrrolo[3,2-d]pyriMidine](/CAS/GIF/129872-83-9.gif)
129872-83-9
7 suppliers
inquiry
Yield:129872-83-9 77%
Reaction Conditions:
Stage #1: 5-benzyl-4-chloro-5H-pyrrolo[3,2-d]pyrimidin-2-aminewith antimony(III) chloride in 1,2-dichloro-ethane at -10; for 0.0833333 h;
Stage #2: with tert.-butylnitrite in 1,2-dichloro-ethane at -10 - 20; for 5 h;
Steps:
11
Example 115-benzyl-2,4-dichloro-5H-pyrrolo[3,2-rfJpyrimidineTo a suspension of 5-ben2yl-4-chloro-5H-pyrrolo[3,2-d]pyrimidin-2-amine (641 mg, 2.5 mmol) in 1,2-dichloroethane (35 mL) was cooled to -10 0C SbCl3 (850 mg, 3.7 mmol) was added. The reaction mixture was stirred for 5 min. ^-butyl nitrite (2.1 mL, 17.4 mmol) was added dropwise and the stirred mixture was from -10 0C to room temperature for 5 h. Upon completion of the reaction, the reaction mixture was poured into ice water and washed with CH2Cl2. The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The crude product was purified by silica gel column chromatography eluting with Hexanes:Ethyl acetate (9:1-1:1), and gave the product as an off- white solid (528 mg, 77%).
References:
WO2006/122003,2006,A2 Location in patent:Page/Page column 33; 43

100-39-0
430 suppliers
$10.00/10g
![2,4-DICHLORO-5H-PYRROLO[3,2-D]PYRIMIDINE](/CAS/GIF/63200-54-4.gif)
63200-54-4
256 suppliers
$18.00/1g
![5-benzyl-2,4-dichloro-5H-pyrrolo[3,2-d]pyriMidine](/CAS/GIF/129872-83-9.gif)
129872-83-9
7 suppliers
inquiry

914496-90-5
0 suppliers
inquiry
![5-benzyl-2,4-dichloro-5H-pyrrolo[3,2-d]pyriMidine](/CAS/GIF/129872-83-9.gif)
129872-83-9
7 suppliers
inquiry
4214-85-1
79 suppliers
$49.00/250mg
![5-benzyl-2,4-dichloro-5H-pyrrolo[3,2-d]pyriMidine](/CAS/GIF/129872-83-9.gif)
129872-83-9
7 suppliers
inquiry
3977-29-5
249 suppliers
$6.00/10g
![5-benzyl-2,4-dichloro-5H-pyrrolo[3,2-d]pyriMidine](/CAS/GIF/129872-83-9.gif)
129872-83-9
7 suppliers
inquiry