5-BENZYLOXY-2-MERCAPTOBENZIMIDAZOLE synthesis
- Product Name:5-BENZYLOXY-2-MERCAPTOBENZIMIDAZOLE
- CAS Number:465546-82-1
- Molecular formula:C14H12N2OS
- Molecular Weight:256.32
Yield:465546-82-1 51%
Reaction Conditions:
in ethanol; for 7 h;Reflux;
Steps:
5.1.13. 2-(4-(2-((5-Hydroxy-1H-benzo[d]imidazol-2-yl)thio)ethyl)piperazin-1-yl)-N-(6-methyl-2,4-bis(methylthio)pyridin-3-yl)acetamide(M4)
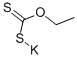
Step 3. Potassium ethylxanthogenate (19.7 g, 123 mmol) was addedto a stirred solution of compound 13 (16.2 g, 75.6 mmol) in EtOH(1000 mL) at rt. The reaction mixture was stirred at reflux for 7 h. Thesolvent was evaporated in vacuo. The residue was diluted with water(1000 mL) and conc. HCl (15 mL) was added to make it acidic(pH = 3). The insoluble material was removed by filtration through apad of Celite. The filtrate was extracted with AcOEt (500 mL × 2). Thecombined organic layers were washed with water (800 mL) and brine(500 mL), and dried over Na2SO4. The solvent was evaporated in vacuo.The residue was purified by silica gel column chromatography (SiO2;1000 g, CHCl3/sat·NH3-MeOH = 100:3) to yield 5-(benzyloxy)-1Hbenzo[d]imidazole-2-thiol (14, 12.9 g, 62%), which was recrystallizedfrom n-hexane/acetone to give pale-yellow crystals (9.95 g, 51%). IR(KBr) cm-1: 3132, 3092, 1635, 1593, 1498, 1452, 1270, 1114, 818; 1HNMR (DMSO-d6) δ: 3.37 (1H, br s), 5.09 (2H, s), 6.75 (1H, d,J = 2.4 Hz), 6.80 (1H, dd, J = 8.5, 2.4 Hz), 7.03 (1H, d, J = 8.5 Hz),7.28-7.46 (5H, m), 12.38 (1H, d, J = 11.7 Hz).
References:
Miura, Toru;Ohgiya, Tadaaki;Omichi, Kozo;Shibuya, Kimiyuki;Tsunenari, Yoshihiko [Bioorganic and medicinal chemistry,2020,vol. 28,# 11]
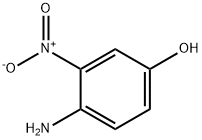
610-81-1
350 suppliers
$5.00/5g

465546-82-1
14 suppliers
inquiry
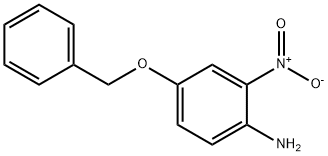
26697-35-8
39 suppliers
$102.50/250mg

465546-82-1
14 suppliers
inquiry

100-39-0
430 suppliers
$10.00/10g

465546-82-1
14 suppliers
inquiry