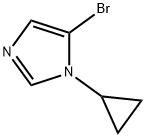
5-broMo-1-cyclopropyl-1H-iMidazole synthesis
- Product Name:5-broMo-1-cyclopropyl-1H-iMidazole
- CAS Number:1262035-61-9
- Molecular formula:C6H7BrN2
- Molecular Weight:187.04
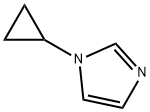
135207-17-9

1262035-61-9
General procedure for the synthesis of 5-bromo-1-cyclopropyl-1H-imidazole from 1-cyclopropyl-1H-imidazole: In a 350 mL four-neck flask, 1-cyclopropyl-1H-imidazole (2.58 g, 23.9 mmol, 1.00 equiv.) was mixed with dichloromethane (100 mL) to form a colorless solution. 1,3-Dibromo-5,5-dimethylimidazolidine-2,4-dione (3.48 g, 12.2 mmol, 0.51 eq.) dissolved in dichloromethane (100 mL) was added slowly and dropwise at 5°C and the reaction mixture was stirred for 2.5 hr. at 0°C to 5°C. Post-treatment: the reaction mixture was burst with 100 mL of sodium sulfite solution. The reaction mixture was extracted with dichloromethane (2 x 200 mL). The organic layer was washed with water/sodium chloride solution, dried with sodium sulfate and concentrated. The crude product was purified by rapid chromatography (silica gel, 120 g, 25% to 100% ethyl acetate in heptane solution) to give 1.21 g of the title compound as a colorless oil.MS (ESI): 187.9/189.1 [M + H]+.

135207-17-9
40 suppliers
$45.00/10mg

1262035-61-9
25 suppliers
$288.00/250 mg
Yield: 37%
Reaction Conditions:
with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione in dichloromethane at 5 - 10; for 2 h;
Steps:
11.2
Step 2 Cyclopropyl-1H-imidazole (1.18 g, 10.9 mmol) was dissolved in methylene chloride (0.1 M), cooled to 5-10° C., and 1,3-dibromo-5,5-dimethylhydantoin (1.59 g, 5.57 mmol) was added. The temperature was maintained between 5-10° C. and the reaction was stirred for 2 hours. Solvent was removed from the reaction mixture under reduced pressure, and the residue was purified by column chromatography (eluding with 1%-7% methanol in methylene chloride, 5% methanol/methylene chloride) to afford 620 mg (37% yield) of 5-bromo-1-cyclopropyl-1H-imidazole.
References:
Gilead Sciences, Inc. US2011/9410, 2011, A1 Location in patent:Page/Page column 30