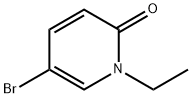
5-BroMo-1-ethylpyridin-2(1h)-one synthesis
- Product Name:5-BroMo-1-ethylpyridin-2(1h)-one
- CAS Number:63785-87-5
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05
13466-38-1

75-03-6

63785-87-5
At room temperature, 5-bromo-1,2-dihydropyridin-2-one (1.04 g, 6 mmol) was dissolved in DMF (12 mL), sodium hydride (60% dispersion in mineral oil, 960 mg, 24 mmol) was added and the reaction mixture was stirred for 30 minutes. Subsequently, ethyl iodide (0.58 mL, 7.2 mmol) was slowly added to the reaction system and stirring was continued overnight at room temperature. This operation was repeated twice to ensure complete reaction. Upon completion of the reaction, the mixture was diluted with water and dichloromethane (DCM). The organic layer was separated, washed sequentially with saturated sodium bicarbonate solution and brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. Finally, the residue was purified by silica gel column chromatography (eluent: DCM/MeOH 98/2) to afford the target compound 5-bromo-1-ethylpyridin-2(1H)-one (532 mg, 2.63 mmol, 44% yield).
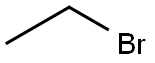
74-96-4
442 suppliers
$9.00/5g

13466-38-1
398 suppliers
$6.00/5g

63785-87-5
47 suppliers
$15.00/250mg
Yield:63785-87-5 74%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 18 - 25;
Steps:
23.D [257j Step D. Preparation of 5-bromo-1-ethylpyridin-2(1H)-one.
To a solution of 5-bromopyridin-2(1H)-one (1.0 g, 6.0 mmol) and K2C03 (2.0 g, 15 mmol) in DMF (10 mL) was added ethyl bromide (0.7 g, 7.5 mmol). The resulting mixture wasstirred overnight at room temperature. Afterward, the mixture was diluted with ethyl acetate (50 mL), washed with H20 (50 mL) and brine (100 mL), dried over Na2SO4 and concentrated. The residue was purified by silica gel chromatography eluting with petroleum ether /ethyl acetate (20/1) to afford 0.9 g (74%) of the title product as a brown oil. LC/MS: mlz 202 [M+H]
References:
WO2016/46260,2016,A1 Location in patent:Paragraph 257

13466-38-1
398 suppliers
$6.00/5g

75-03-6
369 suppliers
$11.00/5g

63785-87-5
47 suppliers
$15.00/250mg

13466-38-1
398 suppliers
$6.00/5g

75-03-6
369 suppliers
$11.00/5g

63785-87-5
47 suppliers
$15.00/250mg
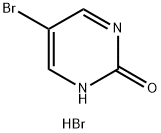
81590-30-9
21 suppliers
inquiry

75-03-6
369 suppliers
$11.00/5g

63785-87-5
47 suppliers
$15.00/250mg