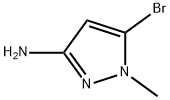
5-Bromo-1-methyl-1H-pyrazol-3-amine synthesis
- Product Name:5-Bromo-1-methyl-1H-pyrazol-3-amine
- CAS Number:89088-55-1
- Molecular formula:C4H6BrN3
- Molecular Weight:176.01
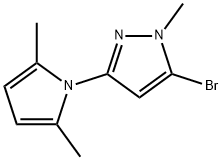
89088-51-7

89088-55-1
Step 3: To a solution of hydroxylamine hydrochloride (3.06 g, 44.1 mmol) in ethanol (20 mL) was sequentially added a mixed water-ethanol (1:1, 40 mL) solution of potassium hydroxide (1.26 g, 22.5 mmol) and a solution of compound 63 (2.3 g, 9 mmol). The reaction mixture was heated to reflux overnight. Upon completion of the reaction, the solvent was removed by concentration under reduced pressure. The resulting residue was purified by silica gel column chromatography (eluent: ethyl acetate/petroleum ether=1:5) to afford compound 64 (1.45 g, 75% yield) as a white solid. lC-MS (Method D): tR=0.58 min, [M+H]+=175.99, 178.01. 1H-NMR (300 MHz, DMSO-d6): δ 3.44 (d, J=93.5 Hz, 3H), 4.77 (s, 2H), 5.53 (s, 1H).

89088-51-7
13 suppliers
inquiry

89088-55-1
59 suppliers
$39.00/100mg
Yield:89088-55-1 75%
Reaction Conditions:
with hydroxylamine hydrochloride;potassium hydroxide in ethanol;waterReflux;
Steps:
1-10.3
Step 3; To a solution of hydroxylamine hydrochloride (3.06 g) in ethanol (20 mL), a solution of potassium hydroxide (1.26 g) in water-ethanol (1:1, 40 mL), and 63 (2.3 g, 9 mmol) were added. The solution was then refluxed overnight. The reaction mixture was then concentrated. The resulting residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether 1:5) to yield 64 as a white solid (1.45 g, y. 75%). LC/MS (Method D): 0.58 min, [M+H]+ = 175.99, 178.01. 1H-NMR (300 Mz) (DMSO): 3.44 (d, J = 93.5 Hz, 3H), 4.77 (s, 2H), 5.53 (s, 1H).
References:
Shionogi & Co., Ltd. EP2426135, 2012, A1 Location in patent:Page/Page column 117