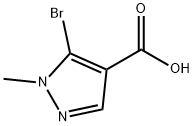
5-Bromo-1-methyl-1H-pyrazole-4-carboxylic acid synthesis
- Product Name:5-Bromo-1-methyl-1H-pyrazole-4-carboxylic acid
- CAS Number:54367-67-8
- Molecular formula:C5H5BrN2O2
- Molecular Weight:205.01
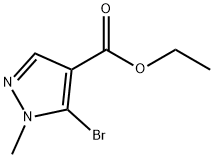
105486-72-4
79 suppliers
$21.00/100mg

54367-67-8
39 suppliers
$58.00/250mg
Yield:54367-67-8 92%
Reaction Conditions:
Stage #1: 5-bromo-1-methyl-1H-pyrazole-4-carboxylic acid ethyl esterwith lithium hydroxide monohydrate;water in tetrahydrofuran;ethanol at 20; for 4 h;
Stage #2: with hydrogenchloride in water; pH=2.5;
Steps:
3.2
Step 2. Synthesis of 5-bromo-1-methyl-1H-pyrazole-4-carboxylic acid A suspension of ethyl 5-bromo-1-methyl-1H-pyrazole-4-carboxylate (8.00 g, 34.3 mmol) in tetrahydrofuran (60 mL), water (20 mL) and ethanol (20 mL) was treated with lithium hydroxide monohydrate (3.17 g, 75.5 mmol) and stirred for 4 hours at room temperature. Removal of solvents under reduced pressure provided a white solid residue, which was diluted with water (50 mL), washed with diethyl ether (50 mL) and adjusted to pH 2.5 with aqueous 6 N hydrochloric acid. The thick suspension was extracted with 2-methyltetrahydrofuran (2*125 mL), and the combined organic layers were dried over magnesium sulfate, filtered and concentrated in vacuo to provide the product as an off-white solid. Yield: 6.49 g, 31.7 mmol, 92%. LCMS m/z 205.2 (M+1). 1H NMR (500 MHz, DMSO-d6) δ 3.86 (s, 3H), 7.91 (s, 1H), 12.64 (br s, 1H).
References:
US2012/214791,2012,A1 Location in patent:Page/Page column 23
31037-02-2
285 suppliers
$6.00/5g

54367-67-8
39 suppliers
$58.00/250mg