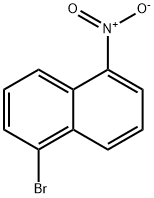
5-bromo-1-nitro-naphthalene synthesis
- Product Name:5-bromo-1-nitro-naphthalene
- CAS Number:5328-76-7
- Molecular formula:C10H6BrNO2
- Molecular Weight:252.06
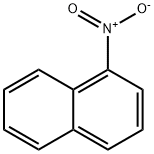
86-57-7

5328-76-7
The general procedure for the synthesis of 1-bromo-5-nitronaphthalene from 1-nitronaphthalene is as follows: 77.5 g (0.485 mol) of bromine was added slowly and dropwise to a stirred mixture of 86 g (0.5 mol) of 1-nitronaphthalene and 0.8-1.0 g of reduced iron powder over a period of 30 min, while the reaction temperature was maintained at 80-85°C. The reaction was carried out in the following manner. After about one hour, the reaction mixture crystallized directly when gas escape ceased. Subsequently, one crystallization was carried out with ethanol and two more with Sorit A from acetone. An average of 52.0 g of a fine yellow needle-like product was obtained with a melting point of 121-122°C (41% yield); the literature reports a melting point of 122.5°C.

86-57-7
20 suppliers
$14.00/5g

5328-76-7
76 suppliers
$5.00/100mg
Yield:5328-76-7 42%
Reaction Conditions:
with ferric(III) chloride;bromine at 90; for 3 h;
Steps:
5.1 A compound represented by [Formula 5-a] was synthesized by the following [Scheme 13].
In a dried 500 mL reactor, 1-nitronaphthalene 100 g (577 mmol) and iron chloride 90 g (58 mmol) were added, and the temperature was raised to 90°C. Then, bromine 93.3 g (577 mmol) was slowly dropped and stirred for 3 hours. .When the reaction was complete, extraction was performed with ethyl acetate and water, the organic layer was concentrated under reduced pressure, and then separated by column chromatography to obtain 61 g of a compound represented by [Formula 5-a]. (Yield 42%)
References:
KR2015/43571,2015,A Location in patent:Paragraph 0390-0396
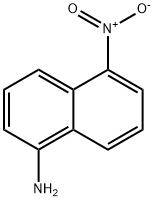
3272-91-1
37 suppliers
inquiry

5328-76-7
76 suppliers
$5.00/100mg