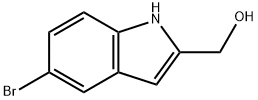
(5-BROMO-1H-INDOL-2-YL)METHANOL synthesis
- Product Name:(5-BROMO-1H-INDOL-2-YL)METHANOL
- CAS Number:53590-48-0
- Molecular formula:C9H8BrNO
- Molecular Weight:226.07
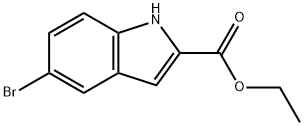
16732-70-0
319 suppliers
$6.00/1g

53590-48-0
75 suppliers
$45.00/50mg
Yield:53590-48-0 97%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 2 h;Inert atmosphere;
Steps:
1.1 Step-1: Preparation of (5-bromo-lH-indol-2-yl)methanol (2)
To a solution of ethyl 5-bromo-lH-indole-2-carboxylate (1, 5.0 g, 18.6 mmol) in THF (35 mL) was added solution of 1 M lithium aluminum hydride in THF (37.2 mL, 37.2 mmol) at 0 °C, dropwise over 20 min. Reaction mixture was stirred for 2 h at rt under N2atmosphere (Reaction condition a). To the reaction mixture ice cold aq.NH4Cl (20 mL) was added drop wise at 0 °C, diluted with water and extracted with ethyl acetate (2 X 200 mL). Organic layer was dried using Na2S04and evaporated. The crude was purified by gradient column chromatography using 20% ethyl acetate in hexane to afford pink coloured solid (2) (4.1 g, 97% yield). MS (ESI): Mass calcd. for C9H8BrNO, m/z 226.07 found 228.0[M+H]2+.
References:
WO2019/77631,2019,A1 Location in patent:Paragraph 000133; 000332
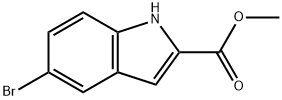
210345-56-5
84 suppliers
$75.00/100mg

53590-48-0
75 suppliers
$45.00/50mg
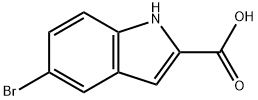
7254-19-5
340 suppliers
$9.00/250mg

53590-48-0
75 suppliers
$45.00/50mg