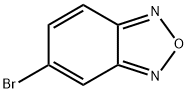
5-Bromobenzo[c][1,2,5]oxadiazole synthesis
- Product Name:5-Bromobenzo[c][1,2,5]oxadiazole
- CAS Number:51376-06-8
- Molecular formula:C6H3BrN2O
- Molecular Weight:199
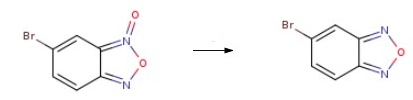
![5-broMobenzo[c][1,2,5]oxadiazole oxide](/CAS/GIF/36387-84-5.gif)
36387-84-5
11 suppliers
inquiry
![5-Bromobenzo[c][1,2,5]oxadiazole](/CAS/GIF/51376-06-8.gif)
51376-06-8
124 suppliers
$29.00/1g
Yield:51376-06-8 71%
Reaction Conditions:
with triethyl phosphite in ethanol at 60; for 1 h;Inert atmosphere;
Steps:
To a stirred solution of 5-bromobenzo[c][l,2,5]oxadiazole 1-oxide (6.0 g, 28.436 mmol) in ethanol (60 mL) was added triethyl phosphite (6.2 mL, 34.123 mmol) at RT under an inert atmosphere. The reaction mixture was then heated at 60 0C for 1 h., cooled to RT, diluted with hexane (100 mL) and stirred for 10 min. The precipitated solid was filtered off and the filtrate was concentrated in vacuo to obtain the crude product. The crude material was purified via silica gel column chromatography to afford 5- , bromobenzo[c][l,2,5] oxadiazole (4.0 g, 71%) as a light yellow solid.
References:
WO2009/158393,2009,A1 Location in patent:Page/Page column 100
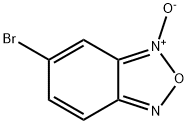
41153-83-7
9 suppliers
inquiry
![5-Bromobenzo[c][1,2,5]oxadiazole](/CAS/GIF/51376-06-8.gif)
51376-06-8
124 suppliers
$29.00/1g
875-51-4
305 suppliers
$5.00/1g
![5-Bromobenzo[c][1,2,5]oxadiazole](/CAS/GIF/51376-06-8.gif)
51376-06-8
124 suppliers
$29.00/1g
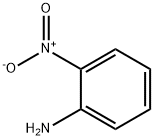
88-74-4
59 suppliers
$10.00/1g
![5-Bromobenzo[c][1,2,5]oxadiazole](/CAS/GIF/51376-06-8.gif)
51376-06-8
124 suppliers
$29.00/1g
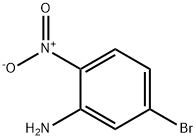
5228-61-5
100 suppliers
$9.00/1g
![5-Bromobenzo[c][1,2,5]oxadiazole](/CAS/GIF/51376-06-8.gif)
51376-06-8
124 suppliers
$29.00/1g