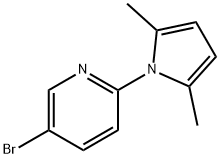
5-bromo-2-(2,5-dimethyl-1H-pyrrol-1-yl)pyridine synthesis
- Product Name:5-bromo-2-(2,5-dimethyl-1H-pyrrol-1-yl)pyridine
- CAS Number:228710-82-5
- Molecular formula:C11H11BrN2
- Molecular Weight:251.12
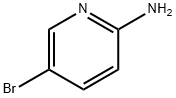
1072-97-5
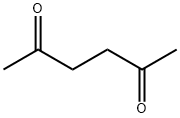
110-13-4

228710-82-5
2-Amino-5-bromopyridine (13.8 g, 0.08 mol) and 2,5-hexanedione (14.1 mL, 0.12 mol) were dissolved in toluene (180 mL) and p-toluenesulfonic acid (100 mg) was added as catalyst. The reaction mixture was placed under reflux for 14 hours under a Dean-Stark apparatus. After completion of the reaction, it was cooled to room temperature and the brown reaction solution was poured into water (200 mL) and extracted with toluene (2 x 200 mL). The organic phases were combined, washed with brine (50 mL), dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography with the eluent of ethyl acetate:pentane (1:3) to afford 5-bromo-2-(2,5-dimethyl-1H-pyrrol-1-yl)pyridine as a brown oil (18.4 g, 0.073 mol, 92% yield). The structure of the product was confirmed by 1H NMR (CDCl3, 400 MHz): δ 2.18 (s, 6H), 5.90 (s, 2H), 7.11 (d, 1H), 7.92 (d, 1H), 8.62 (s, 1H). Low resolution mass spectrometry (LRMS) shows m/z 253 ([M-H]+, Br isotope included).

1072-97-5
702 suppliers
$9.00/1g

110-13-4
399 suppliers
$10.00/5g

228710-82-5
58 suppliers
$50.00/250mg
Yield:228710-82-5 92%
Reaction Conditions:
with toluene-4-sulfonic acid in toluene; for 14 h;Heating / reflux;
Steps:
38 EXAMPLE 38; 5-BROMO-2-F2, 5-DIMETHYLPYRROL-YL PYRIDINE
5-Bromopyridin-2-yl-amine (13. 8g, 0. 08MOL), acetonylacetone (14. 1 ml, 0. 12mol) and p-toluenesulphonic acid (100MG) were dissolved in toluene (180ML) and REFLUXED under Dean Stark conditions for 14 hours. After cooling, the brown solution was poured into water (200MOI) and extracted with toluene (2 x 200ml). The organic extracts were combined and washed with brine (50MI) then dried over anhydrous magnesium sulphate, filtered and concentrated in vacuo to give crude product. This was purified by column chromatography on silica eluting with ethyl acetate: pentane (1: 3) to give the title compound as a brown oil (18. 4g, 0. 073MOL, 92%). 1H NMR (CDC13, 400MHZ) 8 : 2.18 (s, 6H), 5.90 (s, 2H), 7.11 (d, 1 H), 7.92 (d, 1 H), 8.62 (s, 1 H). LRMS: M/Z 253 (M-H+, Br isotope).
References:
WO2004/52372,2004,A1 Location in patent:Page 85-86

1072-97-5
702 suppliers
$9.00/1g
7440-44-0
352 suppliers
$12.00/25g

110-13-4
399 suppliers
$10.00/5g

228710-82-5
58 suppliers
$50.00/250mg