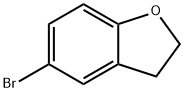
5-Bromo-2,3-dihydro-1-benzofuran synthesis
- Product Name:5-Bromo-2,3-dihydro-1-benzofuran
- CAS Number:66826-78-6
- Molecular formula:C8H7BrO
- Molecular Weight:199.04
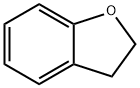
496-16-2

66826-78-6
General procedure for the synthesis of 5-bromo-2,3-dihydrobenzofuran from 2,3-dihydrobenzofuran: Liquid bromine (2.66 g) was added slowly and dropwise to dioxane (30 mL) under cooling conditions in an ice bath and stirred for 30 min at this temperature. Subsequently, 2,3-dihydrobenzofuran (2 g) was added dropwise to the reaction mixture and stirring was continued for 3 hours at room temperature. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was dissolved in ethyl acetate (50 mL), washed sequentially with saturated aqueous sodium bicarbonate and brine, and dried over anhydrous magnesium sulfate. The solvent was removed by evaporation to give an orange oily crude product. The crude product was purified by silica gel column chromatography (eluent: hexane-ethyl acetate, 20:1) to afford 2.33 g of 5-bromo-2,3-dihydrobenzofuran white crystals in 70.3% yield. The product was characterized by 1H-NMR (300 MHz, CDCl3, δ): 3.11 (2H, t, J = 11 Hz), 4.57 (2H, t, J = 11 Hz), 6.66 (1H, d, J = 8 Hz), 7.20 (1H, d, J = 8 Hz), 7.30 (1H, s).
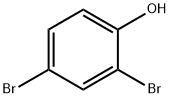
615-58-7
212 suppliers
$10.00/500mg

66826-78-6
152 suppliers
$12.00/1g
Yield:-
Steps:
Multi-step reaction with 2 steps
1: 59 percent / sodium hydroxide / H2O / 22 h / Heating
2: 79 percent Chromat. / BuLi / tetrahydrofuran; hexane / 0.5 h / -100 - -95 °C
References:
Bradsher, Charles K.;Reames, David C. [Journal of Organic Chemistry,1981,vol. 46,# 7,p. 1384 - 1388]

496-16-2
367 suppliers
$7.00/5g

66826-78-6
152 suppliers
$12.00/1g
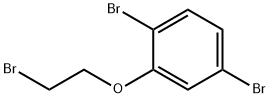
377091-18-4
28 suppliers
$254.09/250mg

66826-78-6
152 suppliers
$12.00/1g
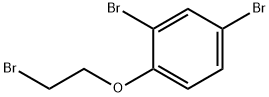
76429-66-8
12 suppliers
$354.82/1g

66826-78-6
152 suppliers
$12.00/1g
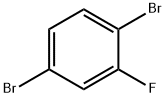
1435-52-5
258 suppliers
$7.00/10g

66826-78-6
152 suppliers
$12.00/1g