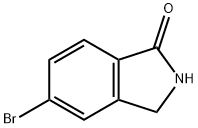
5-BROMO-2,3-DIHYDRO-ISOINDOL-1-ONE synthesis
- Product Name:5-BROMO-2,3-DIHYDRO-ISOINDOL-1-ONE
- CAS Number:552330-86-6
- Molecular formula:C8H6BrNO
- Molecular Weight:212.04
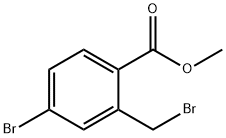
78471-43-9

552330-86-6
The general procedure for the synthesis of 5-bromo-2,3-dihydroisoindol-1-one from 4-bromo-2-bromomethylbenzoic acid methyl ester was as follows: 4-bromo-2-bromomethylbenzoic acid methyl ester (3.70 g) was suspended in a methanol solution of 2 M ammonia (36 mL) and concentrated ammonium hydroxide (12 mL) and the reaction was carried out for 18 hours. After completion of the reaction, the solid product was collected by filtration to afford 5-bromo-2,3-dihydroisoindol-1-one as a colorless solid (2.3 g, 90% yield). The product was characterized by 1H NMR (300 MHz, CDCl3): δ 7.68 (m, 3H), 4.44 (s, 2H).

78471-43-9
229 suppliers
$6.00/250mg

552330-86-6
196 suppliers
$11.00/1g
Yield:552330-86-6 90%
Reaction Conditions:
with ammonia in methanol;water; for 18 h;
Steps:
25
[0189] 4-Bromo-2-bromomethyl-benzoic acid methyl ester (3.70g) was suspended in 2M NH3 in MeOH (36mL) and concentrated ammonium hydroxide (12mL) for eighteen hours. The solid product was filtered to provide the title compound as a colourless solid (2.3 Og, 90%). .H NMR (300MHz, CDCI3): 6 7.68 (m, 3H), 4.44 (s, 2H).
References:
WO2006/20879,2006,A1 Location in patent:Page/Page column 66-67
68837-59-2
351 suppliers
$5.00/1g

552330-86-6
196 suppliers
$11.00/1g
201230-82-2
1 suppliers
inquiry
10269-01-9
201 suppliers
$8.00/1g

552330-86-6
196 suppliers
$11.00/1g
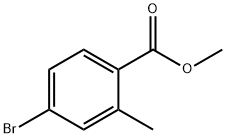
99548-55-7
171 suppliers
$6.00/1g

552330-86-6
196 suppliers
$11.00/1g
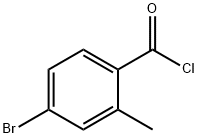
21900-45-8
19 suppliers
inquiry

552330-86-6
196 suppliers
$11.00/1g