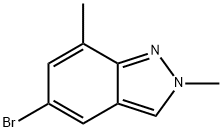
5-bromo-2,7-dimethyl-2H-indazole synthesis
- Product Name:5-bromo-2,7-dimethyl-2H-indazole
- CAS Number:1146637-10-6
- Molecular formula:C9H9BrN2
- Molecular Weight:225.09
156454-43-2
168 suppliers
$13.00/250mg

74-88-4
356 suppliers
$15.00/10g

1146637-10-6
57 suppliers
$45.00/10mg
Yield:-
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;mineral oil at 20; for 0.25 h;
Steps:
95.1 (Step 1)
(Step 1)
Sodium hydride (60% in oil, 240 mg) and iodoethane (0.64 mL) were added to a solution of 5-bromo-7-methyl-1H-indazole (844 mg) in DMF (10 mL), and the reaction solution was stirred at room temperature for 15 minutes.
Water was added to the reaction solution, and then the reaction solution was extracted with ethyl acetate.
The organic layer was washed successively with water and a saturated saline solution.
After drying over anhydrous sodium sulfate, the solvent was evaporated under vacuum.
The resultant residue was purified by column chromatography on silica gel (developing solvent: hexane/ethyl acetate) to obtain 5-bromo-1-ethyl-7-methyl-1H-indazole and 5-bromo-2-ethyl-7-methyl-2H-indazole. In accordance with Example 70 (Step 1), iodomethane was used instead of iodoethane to obtain 5-bromo-2,7-dimethyl-2H-indazole.
References:
Taiho Pharmaceutical Co., Ltd.;SAKAMOTO, Toshihiro;MITA, Takashi;SHIBATA, Kazuaki;OGINO, Yoshio;KOMATANI, Hideya EP2762476, 2014, A1 Location in patent:Paragraph 0270; 0320