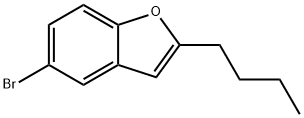
5-BroMo-2-butyl-benzofuran synthesis
- Product Name:5-BroMo-2-butyl-benzofuran
- CAS Number:497225-66-8
- Molecular formula:C12H13BrO
- Molecular Weight:253.13
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

497225-66-8
1 suppliers
inquiry
Yield: 67%
Reaction Conditions:
with triethylamine;benzenesulfonyl chloride in water;toluene at 80;
Steps:
C C. 2-n-Butyl-5-bromobenzofuran (Compound IX: R1=n-C4-H9; R2=H; X1=Br)
C.
2-n-Butyl-5-bromobenzofuran (Compound IX: R1=n-C4-H9; R2=H; X1=Br)
25.8 ml of benzenesulfonyl chloride (0.202 mol; 1.4 equivalents) and 40 ml of toluene are placed in an equipped reactor and the mixture is stirred at 80° C. 65 ml of anhydrous triethylamine (0.47 mol) and then 45.2 g of 2-(4-bromo-2-formylphenoxy)hexanoic acid (compound XI) (0.144 mol) dissolved in 250 ml of toluene are then added slowly at 80° C.
The reaction progress is monitored by TLC (eluent: 80/20 methylcyclohexane/ethyl acetate; Rf of compound XI=0.08; Rf of the desired compound XII=0.80).
At the end of the reaction, the temperature of the reaction medium is returned to 20° C.
The excess benenesulfonyl chloride is destroyed by addition of 250 ml of aqueous 5% sodium hydroxide solution.
The phases are decanted and separated and the organic phase is then washed with a mixture of 70 ml of deionized water and 6.8 ml of 37% hydrochloric acid.
The phases are decanted and separated and the organic phase is then washed with 75 ml of deionized water.
The organic phase is washed with a solution of 7.73 g of sodium hydroxide dissolved in 67 ml of deionized water.
The phases are decanted and separated and the organic phase is then washed with a solution of 7.53 g of sodium chloride in 70 ml of deionized water.
The pH of the aqueous phase is adjusted to between 5 and 8 with 7% hydrochloric acid solution.
The phases are decanted and separated and the organic phase is then concentrated on a rotary evaporator to give 37.2 g of a brown oil.
This oil is purified by chromatography on silica gel (eluent: 80/20 methylcyclohexane/ethyl acetate) to give 24.3 g of the desired compound XII in the form of a yellow oil.
Yield: 67%
1H NMR (400 MHz, DMSO-d6): δ 0.91 (t, J=7.2 Hz, 3H, -CH2-CH2-CH3); 1.30-1.40 (m, 2H, -CH2-CH2-CH3) ; 1.61-1.69 (m, 2H, -CH2-CH2-CH2-); 2.76 (t, J=7.4 Hz, 2H, -CH2-CH2-Cq); 6.57 (s, 1H, ArH); 7.33 (dd, J=8.8 and 2 Hz, 1H, ArH); 7.46 (d, J=8.8 Hz, 1H, ArH); 7.72 (dd, 2 Hz, 1H, ArH)
References:
SANOFI;Priem, Thomas;Vayron, Philippe Paul US2013/23678, 2013, A1 Location in patent:Paragraph 0061-0065
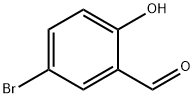
1761-61-1
386 suppliers
$5.00/5g

497225-66-8
1 suppliers
inquiry
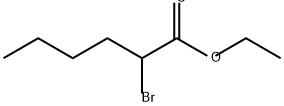
615-96-3
140 suppliers
$27.00/1g

497225-66-8
1 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

497225-66-8
1 suppliers
inquiry