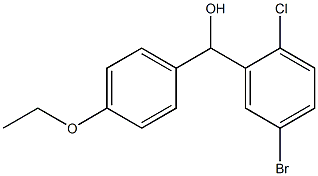
(5-bromo-2-chlorophenyl)(4-ethyloxyphenyl)methanol synthesis
- Product Name:(5-bromo-2-chlorophenyl)(4-ethyloxyphenyl)methanol
- CAS Number:1280647-32-6
- Molecular formula:C15H14BrClO2
- Molecular Weight:341.63
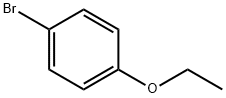
588-96-5
298 suppliers
$7.00/5g
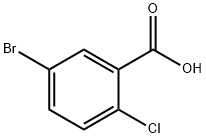
21739-92-4
671 suppliers
$8.00/10g

1280647-32-6
37 suppliers
inquiry
Yield:1280647-32-6 225 g
Reaction Conditions:
Stage #1: 4-bromoethoxybenzenewith n-butyllithium in tetrahydrofuran;hexane at -80 - -70; for 0.5 h;
Stage #2: 5-bromo-2-chlorobenzoic acid in tetrahydrofuran;hexane at -90 - -80;
Steps:
3 EXAMPLE-3: Preparation of 5-halo-2-chloro-l-(4-ethoxy phenyl) methanol compound of Formula VI-I (wherein X=bromo).
4-bromo phenetole (398.53 gms) and tetrahydrofuran (3000 ml) were added into a round bottom flask at 25-35°C and allowed to cool to -70 to -80°C. To this solution, n-butyl lithium in hexane (1.6M, 854.34 ml) was added slowly at -70 to -80°C and stirred for 30 mins. Compound of Formula II (wherein X=bromo, 300gms) in tetrahydrofuran (900 ml) was added to the above reaction mass slowly at -80 to -90°C and maintained for 2-4 hrs at the same temperature. The reaction mass was treated with aq ammonium chloride solution (0.5 gms of ammonium chloride in 5 ml of water), stirred for 30 mins at 25-35°C. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. Combined the organic layer, washed with water and concentered the organic layer up to 300 ml under reduced pressure at below 50°C. The resulting reaction mass was co-distilled with cyclohexane (900 ml) up to 300 ml twice and cooled the reaction mass to 25-35°C. 10% IPA in cyclohexane (900 ml) was added to the above reaction mass at 25-35°C and raised the reaction mass temperature to 70- 80°C to get clear solution. The solution was cooled to 25-35°C and stirred for 4-5hrs, filtered, washed with cyclohexane and dried the material at 50-55°C. HPLC analysis revealed the content of impurity of Formula A: about 4 to 5%; impurity of Formula B: about 2 to 3%; impurity of Formula C: about 0.5 % and impurity of Formula D: about 0.5 to 1%. 10% IPA in cyclohexane (900 ml) was added to the above material at 25-35°C, heated to 70- 80°C and maintained for 15-30 mins. The resulting solution was cooled to 25-35°C and maintained for 4hrs, filtered, washed with cyclohexane and dried at 50-55°C under vacuum for 4 hrs to obtain the title compound. Yield: 225 gms; HPLC Purity: 99.0%; impurity of Formula A: less than 0.5%; impurity of Formula B: less than 0.1%; impurity of Formula C: less than 0.1% and impurity of Formula D: less than 0.2%.
1H NMR (300 MHz, CDC13) δ = 7.17 (d, 1H), 7.33 (dd, 1H), 7.86 (d, 1H), 6.06 (d, 1H), 7.26 (m, 2H), 6.84 (m, 2H), 4.01 (q, 2H), 1.39 (t, 3H), 2.30 (d, OH); MS (ESI)[M+H]:- 340 The PXRD is set forth in Figure 1.
References:
WO2018/29611,2018,A1 Location in patent:Page/Page column 60-61
461432-22-4
350 suppliers
$5.00/250mg

1280647-32-6
37 suppliers
inquiry

21739-92-4
671 suppliers
$8.00/10g

1280647-32-6
37 suppliers
inquiry

21900-52-7
86 suppliers
$12.00/250mg

1280647-32-6
37 suppliers
inquiry
103-73-1
354 suppliers
$8.00/25g

1280647-32-6
37 suppliers
inquiry