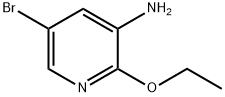
5-Bromo-2-ethoxypyridin-3-amine synthesis
- Product Name:5-Bromo-2-ethoxypyridin-3-amine
- CAS Number:886373-00-8
- Molecular formula:C7H9BrN2O
- Molecular Weight:217.06

886372-76-5

886373-00-8
The general procedure for the synthesis of 5-bromo-2-ethoxypyridin-3-amine from 5-bromo-2-ethoxy-3-nitropyridine was as follows: 5-bromo-2-ethoxy-3-nitropyridine (75.2 mg, 0.304 mmol) was dissolved in ethyl acetate (3 mL), and stannous chloride (II) (289 mg, 1.52 mmol) was added. The reaction mixture was heated to reflux and maintained for 2 hours. Upon completion of the reaction, it was cooled to room temperature and 50% aqueous sodium hydroxide solution was added slowly and dropwise until a viscous brown solid formed in the reaction mixture. Subsequently, anhydrous sodium sulfate was added and stirred for several minutes. Solid impurities were removed by filtration and the filtrate was dried with anhydrous sodium sulfate, filtered again and concentrated under reduced pressure to afford 5-bromo-2-ethoxypyridin-3-amine (53 mg, 0.25 mmol, 80% yield) as a dark blue film. The product was characterized by 1H NMR (400 MHz, CDCl3): δ 7.56 (d, 1H), 6.97 (d, 1H), 4.37 (q, 2H), 3.85 (br s, 2H), 1.40 (dd, 3H); the results of mass spectrometry (EI) analysis conformed to the molecular weight of C7H9BrN2O: 217,219 (Br isotope, MH+).

886372-76-5
10 suppliers
inquiry

886373-00-8
39 suppliers
$11.00/250mg
Yield:886373-00-8 80%
Reaction Conditions:
Stage #1: 5-bromo-2-ethoxy-3-nitropyridinewith tin(ll) chloride in ethyl acetate; for 2 h;Reflux;
Stage #2: with sodium hydroxide in water;ethyl acetate at 20;
Steps:
30.2
To a solution of 5-bromo-2-ethoxy-3-nitropyridine (75.2 mg, 0.304 mmol) in ethyl acetate (3 mL) was added tin(II) chloride (289 mg, 1.52 mmol), and the mixture was heated to reflux for 2 h. After cooling to rt, 50% aqueous sodium hydroxide was added dropwise until a sticky brown solid completely formed. Sodium sulfate was then added, and the mixture was stirred for several minutes. The solids were then removed by filtration. The filtrate was dried over sodium sulfate, filtered, and concentrated in vacuo to provide 5-bromo-2-ethoxypyridin-3-amine (53 mg, 0.25 mmol, 80% yield) as a dark blue film. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, 1H), 6.97 (d, 1H), 4.37 (q, 2H), 3.85 (br s, 2H), 1.40 (dd, 3H); MS (EI) for C7H9BrN2O: 217, 219 (Br isotopes, MH+).
References:
WO2010/135524,2010,A1 Location in patent:Page/Page column 237
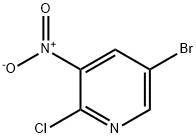
67443-38-3
377 suppliers
$5.00/1g

886373-00-8
39 suppliers
$11.00/250mg