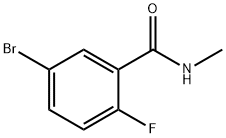
5-bromo-2-fluoro-N-methylbenzamide synthesis
- Product Name:5-bromo-2-fluoro-N-methylbenzamide
- CAS Number:1016777-86-8
- Molecular formula:C8H7BrFNO
- Molecular Weight:232.05
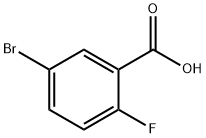
146328-85-0
288 suppliers
$6.00/1g

74-89-5
0 suppliers
$13.44/25ML

1016777-86-8
22 suppliers
inquiry
Yield:1016777-86-8 93%
Reaction Conditions:
Stage #1: 5-bromo-2-fluoro-benzoic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20; for 1.5 h;
Stage #2: methylamine in dichloromethane at 0; for 0.5 h;
Steps:
39.1
To a mixture of 5-bromo-2-fluorobenzoic acid (200 mg, 0.91 mmol) in dichloromethane (5 mL) was added oxalyl chloride (0.16 mL, 1.8 mmol), followed by 1 drop of N,N-dimethylformamide. The reaction was stirred at room temperature for 1.5 hours. The reaction was concentrated and the resulting residue was dissolved in dichloromethane (3 mL) and cooled to 0° C. Methylamine (2.3 mL, 5 mmol, 2 M in tetrahydrofuran) was added and the reaction was allowed to stir at 0° C. for 30 minutes. The reaction was quenched with water and the mixture was concentrated. The residue was diluted with water and the resulting solids were filtered, rinsed with water, and dried under vacuum to give the title compound (196.6 mg, 93%) as a white solid. +ESI (M+H+1) 234.1; 1H NMR (400 MHz, CDCl3, δ); 8.22 (dd, J=6.83, 2.73 Hz, 1H), 7.55 (ddd, J=8.68, 4.49, 2.63 Hz, 1H), 7.00 (dd, J=11.32, 8.58 Hz, 1H), 6.67 (br. s., 1H), 3.02 (dd, J=4.88, 1.17 Hz, 3H).
References:
US2012/108619,2012,A1 Location in patent:Page/Page column 45
773140-42-4
23 suppliers
$75.00/100mg

74-89-5
0 suppliers
$13.44/25ML

1016777-86-8
22 suppliers
inquiry

146328-85-0
288 suppliers
$6.00/1g

1016777-86-8
22 suppliers
inquiry