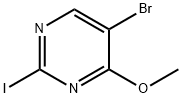
5-Bromo-2-iodo-4-methoxypyrimidine synthesis
- Product Name:5-Bromo-2-iodo-4-methoxypyrimidine
- CAS Number:1443792-51-5
- Molecular formula:C5H4BrIN2O
- Molecular Weight:314.91
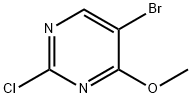
57054-92-9
108 suppliers
$7.00/1g

1443792-51-5
6 suppliers
inquiry
Yield:1443792-51-5 16%
Reaction Conditions:
with hydrogen iodide;sodium iodide in water at 40; for 16 h;
Steps:
5-bromo-2-iodo-4-methoxypyrimidine
Prepared using General Procedure 16: To a stirred solution of 5-bromo- 2-chloro-4-methoxypyrimidine (100 mg, 0.447 mmol) in 57 % aq. HI (1.0 mL) was added sodium iodide (125 mg, 0.838 mmol). The reaction mixture was stirred at 40°C for 16 h, cooled, then quenched with NaHC03 (5 mL) and extracted with EA (3 x 5 mL). The combined organics were washed with brine, dried over MgS04 and concentrated to afford 22.0 mg (16%) of 5-bromo-2-iodo-4-methoxypyrimidine as an off-white solid. LCMS-ESI (m/z) calculated for C5H4BrIN20: 314.9; found 315.9 [M+H]+, tR = 8.22 min. (Method 2). 1H NMR (400 MHz, DMSO) δ 8.25 (s, 1H), 4.07 (s, 3H).
References:
WO2013/90454,2013,A2 Location in patent:Paragraph 00387