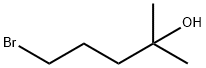
5-Bromo-2-methyl-2-pentanol synthesis
- Product Name:5-Bromo-2-methyl-2-pentanol
- CAS Number:21871-76-1
- Molecular formula:C6H13BrO
- Molecular Weight:181.07
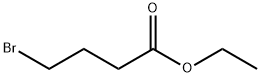
2969-81-5
458 suppliers
$5.00/1g

75-16-1
274 suppliers
$12.00/10ml

21871-76-1
15 suppliers
$155.00/1g
Yield:21871-76-1 67%
Reaction Conditions:
Stage #1: Ethyl 4-bromobutyrate;methylmagnesium chloride in diethyl ether at -20 - 20;Inert atmosphere;
Stage #2: with ammonia hydrochloride
Steps:
1
5-Bromo-2-methyl-2-pentanol (8). To a -20° C. cooled solution of ethyl-4-bromobutyrate 7 (5 g, 25.6 mmol) in anhydrous diethyl ether (50 mL) was added 3M solution of methylmagnesium bromide in diethyl ether (17.1 mL, 6.11 g, 51.3 mmol) under argon atmosphere over a period of 30 min. The reaction mixture was stirred at room temperature for overnight. Saturated ammonium chloride solution was added to hydrolyse the reaction mixture followed by 1N HCl solution to dissolve the inorganic salts formed. The aqueous phase was extracted with ether (3×50 mL). The combined extracts were washed with water (100 mL), saturated NaCl solution (100 mL), dried (Na2SO4), filtered and concentrated. The residue was purified by column chromatography to afford 3.1 g (17.1 mmol, 67%) of tertiary alcohol 8. 1H NMR (400 MHz, CDCl3) δ: 1.27 (6H, s), 1.64 (2H, m), 1.96 (2H, m), 3.44 (2H, t, J=6.68 Hz).
References:
US2010/9941,2010,A1 Location in patent:Page/Page column 4

2969-81-5
458 suppliers
$5.00/1g

21871-76-1
15 suppliers
$155.00/1g
1071-73-4
264 suppliers
$27.00/25g

21871-76-1
15 suppliers
$155.00/1g
3884-71-7
71 suppliers
$64.00/1g
917-54-4
184 suppliers
$52.10/25ml

21871-76-1
15 suppliers
$155.00/1g

3884-71-7
71 suppliers
$64.00/1g

75-16-1
274 suppliers
$12.00/10ml

21871-76-1
15 suppliers
$155.00/1g