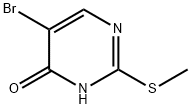
5-bromo-2-methylsulfanyl-3H-pyrimidin-4-one synthesis
- Product Name:5-bromo-2-methylsulfanyl-3H-pyrimidin-4-one
- CAS Number:81560-03-4
- Molecular formula:C5H5BrN2OS
- Molecular Weight:221.07
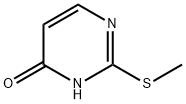
5751-20-2

81560-03-4
Step 2, Preparation of 5-bromo-2-(methylthio)pyrimidin-4(3H)-one: 142 g of 2-methylthio-4-pyrimidinone was dissolved in 500 mL of dichloromethane, 190 g of N-bromosuccinimide (NBS) was added, and the reaction was heated and refluxed for 6 hours. After completion of the reaction, the reaction mixture was cooled to room temperature and filtered to remove insoluble material. The filter cake was washed with an appropriate amount of dichloromethane, and the organic phases were combined and dried with anhydrous sodium sulfate. The dried organic phase was recrystallized under ice bath conditions to give 190 g of light yellow solid product in 87% yield.

5751-20-2
304 suppliers
$14.00/1g

81560-03-4
91 suppliers
$45.00/100mg
Yield: 87%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane for 6 h;Reflux;
Steps:
1.2 Example 1
Step 2, Preparation of 5-bromo-2-methylthio-4-pyrimidinone: Take 142 g of 2-methylthio-4-pyrimidinone, dissolve in 500 ml of methylene chloride, add NBS 190 g, reflux for 6 hours, After the reaction is completed, cool to room temperature, filter, wash the filtrate, organicThe phase was dried and recrystallized on an ice bath to give 190 g of a pale yellow solid in a yield of 87%.
References:
Shanghai Weiju Industrial Co., Ltd.;Liu Hui CN107488175, 2017, A Location in patent:Paragraph 0008; 0010