
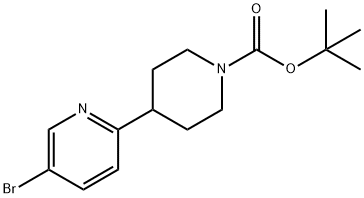
5-broMo-2-(piperidin-4-yl)pyridine synthesis
- Product Name:5-broMo-2-(piperidin-4-yl)pyridine
- CAS Number:845788-60-5
- Molecular formula:C10H13BrN2
- Molecular Weight:241.13
622387-27-3
24 suppliers
inquiry

845788-60-5
16 suppliers
inquiry
Yield:845788-60-5 53%
Reaction Conditions:
with hydrogenchloride;water in 1,4-dioxane;methanol at 20; for 18.25 h;
Steps:
A27.A Part A
To a solution of the Boc-piperidine of Example 24, Part B (8.1 g, 23.8 mmol) in 1,4-dioxane (10 mL) was added 4M HCl in 1,4-dioxane (10 mL). Methanol (5 mL) was then added, and the resulting solution was stirred at ambient temperature for 18 hr. Additional 4M HCl in 1,4-dioxane (10 mL) was added and the reaction was complete in 15 min. The solution was concentrated in vacuo to provide the amine in the form of a yellow solid. To the crude amine suspended into methylene chloride (50 mL) was added triethylamine (8.29 mL, 59.5 mmol). The mixture was cooled to 0° C., and methanesulfonyl chloride (1.75 mL, 22.6 mmol) was added. Afterward, the mixture was stirred for 48 hr at ambient temperature. The mixture was then concentrated in vacuo. The resulting residue was dissolved into ethyl acetate. The organic layer was washed with water, saturated sodium bicarbonate, and saturated NaCl; dried over sodium sulfate; and concentrated in vacuo. Ethyl ether was added, and the resulting white solid was collected by vacuum filtration to provide the desired mesylate intermediate in the form of a white solid (4.0 g, 53% yield). MS MH+ for C11H15BrN2O2S: calc. 319, found 319.
References:
US2005/9838,2005,A1 Location in patent:Page 300-301