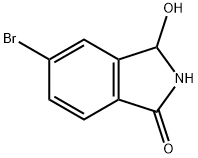
5-bromo-3-hydroxyisoindolin-1-one synthesis
- Product Name:5-bromo-3-hydroxyisoindolin-1-one
- CAS Number:573675-39-5
- Molecular formula:C8H6BrNO2
- Molecular Weight:228.04
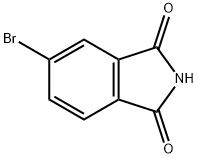
6941-75-9
181 suppliers
$5.00/1g

573675-39-5
22 suppliers
inquiry
Yield:573675-39-5 95%
Reaction Conditions:
Stage #1: 5-Bromoisoindoline-1,3-dionewith sodium hydroxide;copper(II) sulfate;zinc in water at 0 - 20; for 3 h;
Stage #2: with hydrogenchloride in water; pH=7;
Steps:
5.5A
EXAMPLE 5; Step 5A: Bromophthalimide 5a (13.0 g, 57.8 mmol) was added in six portions over 30 minutes to a stirred suspension of zinc powder (4.50 g, 69.2 mmol) and copper (II) sulfate pentahydrate (0.060 g, 0.25 mmol) in aqueous sodium hydroxide (2 M, 71 mL) at 0 °C (ice-bath. ) The mixture was stirred at 0 °C for an additional 30 minutes, and at room temperature for 2.5 h to complete the reaction. After filtering, the reaction solution was neutralized to pH 7 with 20% hydrochloric acid, diluted with 100 mL of ethanol, and then extracted with ethyl acetate. The extract was washed with brine, dried with MgS04 and concentrated in vacuo to afford 12.5 g (95%) of 5b as a yellow solid, LC-MS 210 (MH+-H20.)
References:
WO2005/103039,2005,A1 Location in patent:Page/Page column 32
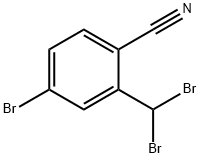
573675-37-3
5 suppliers
inquiry

573675-39-5
22 suppliers
inquiry