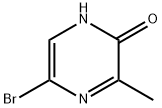
5-BroMo-3-Methylpyrazin-2-ol synthesis
- Product Name:5-BroMo-3-Methylpyrazin-2-ol
- CAS Number:100047-56-1
- Molecular formula:C5H5BrN2O
- Molecular Weight:189.01
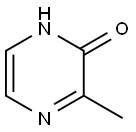
19838-07-4

100047-56-1
The general procedure for the synthesis of 2-hydroxy-3-methyl-5-bromopyrazine from 2-hydroxy-3-methylpyrazine was as follows: to a solution of 3-methylpyrazin-2-ol (1.5 g, 13.62 mmol) in anhydrous N,N-dimethylformamide (DMF, 20 mL) was added batchwise at 0 °C N-bromosuccinimide (NBS, 2.67 g, 14.98 mmol). The reaction mixture was slowly warmed to room temperature and stirred overnight. Upon completion of the reaction, the mixture was poured into ice water and extracted with a solvent mixture of isopropanol (IPA)/dichloromethane (DCM, 1/5, v/v). The organic phases were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography with 80% ethyl acetate/hexane (v/v) as eluent to afford 2-hydroxy-3-methyl-5-bromopyrazine as white solid (1.97 g, 10.42 mmol, 77% yield). The product was analyzed by mass spectrometry (LCMS) with measured m/z = 189.0 [M+H]+ (calculated exact mass of C5H5BrN2O: 188.0).1H NMR (400 MHz, DMSO-d6) δppm: 2.27 (d, J = 0.5 Hz, 3H), 7.73 (s, 1H), 12.3 (s, 1H).

19838-07-4
148 suppliers
$9.00/100mg

100047-56-1
44 suppliers
$27.00/100mg
Yield:100047-56-1 77%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 0 - 20;
Steps:
1.115.A
To a solution of 3-methylpyrazin-2-ol (1.5 g, 13.62 mmol) in anhydrous DMF (20 mL) was added N-bromosuccinimide (2.67 g, 14.98 mmol) at 0 °C. The reaction mixture was slowly warmed to room temperature and stirred overnight. The resulting mixture was poured into water, and extracted with IPA/DCM (1/5). The combined organics were dried over anhydrous Na2S04, filtered then concentrated. The residue was purified by column chomatography with 80% ethyl acetate/hexanes to give the title compound as white solid (1.97 g, 10.42 mmol, 77% yield).Exact mass calculated for C5H5BrN20: 188.0, found: LCMS m/z = 189.0 [M+H]+; lU NMR (400 MHz, DMSO-i ) δ ppm 2.27 (d, J = 0.5 Hz, 3H), 7.73 (s, 1H), 12.3 (s, 1H).
References:
ARENA PHARMACEUTICALS, INC.;JONES, Robert M.;HAN, Sangdon;BUZARD, Daniel J.;LEHMANN, Juerg;NARAYANAN, Sanju;YUE, Dawei WO2011/127051, 2011, A1 Location in patent:Page/Page column 205