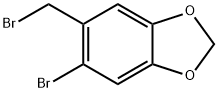
5-BROMO-6-BROMOMETHYL-1 3-BENZODIOXOLE synthesis
- Product Name:5-BROMO-6-BROMOMETHYL-1 3-BENZODIOXOLE
- CAS Number:5434-47-9
- Molecular formula:C8H6Br2O2
- Molecular Weight:293.94
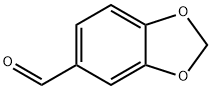
120-57-0

5434-47-9
The general procedure for the synthesis of 5-bromo-6-(bromomethyl)benzo[d][1,3]dioxole from piperonal was as follows: bromine (3.36 g, 21 mmol) was added slowly and dropwise to a solution of piperonal (3.0 g, 20 mmol) in chloroform (15 mL). The reaction mixture was heated to 60 °C and the reaction was stirred at this temperature for 12 hours. Upon completion of the reaction, the mixture was cooled to room temperature and diluted with the addition of dichloromethane (60 mL). Subsequently, the organic phase was washed sequentially with saturated aqueous sodium bicarbonate (2 x 20 mL) and brine (20 mL), and the organic layer was dried with anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure to give the crude product 5-bromo-6-(bromomethyl)benzo[d][1,3]dioxole (5.88 g, 100% yield), which solidified on standing. A portion of the crude product (2.44 g, 8.33 mmol) was taken and dissolved in tetrahydrofuran and the solution was cooled to -78 °C. Under nitrogen protection, lithium aluminum hydride (348 mg, 9.16 mmol) was added in batches and the reaction was stirred at -78 °C for 3 hours. Subsequently, lithium aluminum hydride (80 mg, 2.03 mmol) was added and stirring was continued for 1 hour. Upon completion of the reaction, ethyl acetate was carefully added to quench the excess lithium aluminum hydride, followed by methanol (20 mL). The insoluble salt was removed by filtration and the filtrate was concentrated under reduced pressure to afford the target product 5-bromo-6-(bromomethyl)benzo[d][1,3]dioxole (1.76 g, 99% yield), which could be used in the subsequent reaction without further purification. The product was characterized by 1H-NMR (CDCl3): δ 7.01 (s, 1H), 6.73 (s, 1H), 5.95 (s, 2H), 2.32 (s, 3H).

120-57-0
0 suppliers
$26.35/25g

5434-47-9
27 suppliers
$40.00/1g
Yield:5434-47-9 100%
Reaction Conditions:
with bromine at 60; for 12 h;
Steps:
304.a Example 304; 4-[3-(6-methyl-benzo[1,3]dioxol-5-yl)-benzenesulfonyl]-5-methylsulfanyl-thiophene-2-carboxamidine trifluoroacetate; a); 5-Bromo-6-methyl-benzo[1,3] dioxole
Bromine (3.36 g, 21 mmol) was added to a solution of piperonal alcohol (3.0 g, 20 mmol) in chloroform (15 mL). The solution was heated to 60 oC FOR 12 h. After cooling, DCM (60 mL) was added and the solution was extracted with aq NAHC03 (2 x 20 mL), brine (20 mL), and was dried over sodium sulfate. After concentration, the residue 5-bromo-6-bromomethyl-benzo[1,3] dioxole (5. 88G, 100%), solidified upon standing. A portion of the crude solid (2.44g, 8. 33 mmol) was dissolved in THF and cooled TO-78 oC. Lithium aluminum hydride (348 mg, 9.16 mmol) was added and the solution was stirred for 3 h. An additional portion of LAH (80 mg, 2.03 mmol) was added and the reaction was stirred an additional hour. EtOAc was added carefully to quench the excess LAH, followed by addition OF MEOH (20 mL). The salts were filtered and the filtrate was concentrated in vacuo, to yield the title compound (1.76 g, 99%) which was used without further purification. 1H-NMR (CDC13) : δ 7.01 (s, 1H), 6.73 (s, 1H), 5.95 (s, 2H), 2.32 (s, 3H).
References:
WO2003/99805,2003,A1 Location in patent:Page 418-419
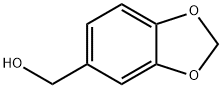
495-76-1
300 suppliers
$15.00/5g

5434-47-9
27 suppliers
$40.00/1g
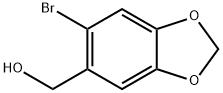
6642-34-8
62 suppliers
$38.00/100mg

5434-47-9
27 suppliers
$40.00/1g
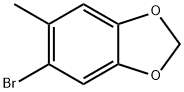
5025-53-6
12 suppliers
inquiry

5434-47-9
27 suppliers
$40.00/1g
15930-53-7
157 suppliers
$5.00/1g

5434-47-9
27 suppliers
$40.00/1g