
5-bromo-6-chloro-1-methylpyridin-2(1H)-one synthesis
- Product Name:5-bromo-6-chloro-1-methylpyridin-2(1H)-one
- CAS Number:960299-33-6
- Molecular formula:C6H5BrClNO
- Molecular Weight:222.47
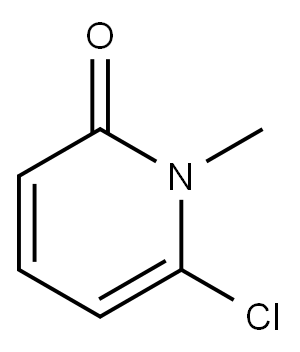
17228-63-6
40 suppliers
$45.00/50mg
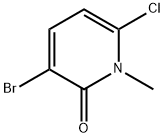
960299-32-5
24 suppliers
inquiry

960299-33-6
10 suppliers
inquiry
Yield:960299-32-5 55% ,960299-33-6 30%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 20; for 2 h;
Steps:
69.A
To a solution of 6-chloro-l-methylpyridin-2(lH)-one (0.500 g, 3.48 mmol; obtained from Example 68, Step A) in DMF (15 mL) was added N-bromosuccinimide (0.620 g, 3.48 mmol). The reaction was stirred at room temperature for 2 hours and then quenched with 10% sodium bisulfite solution. The reaction mixture was partitioned between EtOAc and H2O. The phases were separated, and the aqueous phase was re-extracted with EtOAc (Ix). The combined organic layers were dried (Na2SO4), filtered and concentrated to yield a yellow oil. The crude product was purified by silica gel flash column chromatography, eluting with 20:1 CH2Cl2ZEtOAc. The desired product (0.424 g, 55%) was obtained as a white crystalline solid. 1H-NMR (400 MHz, DMSO-d6) δ 7.90 (d, IH), 6.48 (d, IH), 3.63 (s, 3H). LRMS (ESI pos) m/e 222, 224 (M+, Br pattern). Also isolated was 5-bromo-6-chloro-l-methylpyridin-2(lH)-one (0.233 g, 30%) as a
References:
WO2007/146824,2007,A2 Location in patent:Page/Page column 128; 129

17228-63-6
40 suppliers
$45.00/50mg

960299-33-6
10 suppliers
inquiry
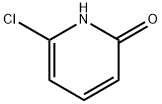
16879-02-0
182 suppliers
$11.00/5g

960299-33-6
10 suppliers
inquiry