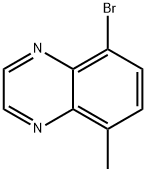
5-Bromo-8-methylquinoxaline synthesis
- Product Name:5-Bromo-8-methylquinoxaline
- CAS Number:1360599-43-4
- Molecular formula:C9H7BrN2
- Molecular Weight:223.07
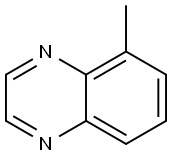
13708-12-8

1360599-43-4
5-Methylquinoxaline (9.50 g, 65.97 mmol) was dissolved in acetonitrile (80 mL) at room temperature, followed by addition of N-bromosuccinimide (27.00 g, 151.74 mmol). The reaction mixture was stirred at 60 °C for 16 hours. Upon completion of the reaction, the mixture was cooled to room temperature and subsequently concentrated under reduced pressure. To the concentrated residue was added ethyl acetate (500 mL) for dilution. Insoluble solids were removed by filtration and the filtrate was sequentially washed with saturated brine and dried with anhydrous sodium sulfate. Finally, the solvent was removed under reduced pressure to afford the target compound 5-bromo-8-methylquinoxaline as a brown solid (6.00 g, 41% yield). Mass spectrometry result: m/z = 222.9 [M + H]+.

13708-12-8
282 suppliers
$11.00/5g

1360599-43-4
24 suppliers
inquiry
Yield:1360599-43-4 41%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 60; for 16 h;
Steps:
5-Bromo-8-methylquinoxaline:
To a solution of 5-methylquinoxaline (9.50 g, 65.97 minol) in CH3CN (80 mL) was added 1-bromopyrrolidine-2,5-dione (27.00 g, 151.74 minol) at room temperature. The resulting solution was stirred for 16 h at 60 °C. After cooling to room temperature, the reactionminxture was concentrated under reduced pressure and the residue was diluted with ethyl acetate (500 mL). The insoluble solids in theminxture were filtered out and the filtrate was washed with brine and dried over Na2SO4. The solvent was removed under reduced pressure to yield 5-bromo-8-methylquinoxaline as brown solid (6.00 g, 41%). MS: m/z = 222.9 [M+Hj .
References:
WO2017/106607,2017,A1 Location in patent:Paragraph 00217; 00218
![4-BROMO-7-METHYL-BENZO[1,2,5]THIADIAZOLE](/CAS/GIF/2255-80-3.gif)
2255-80-3
62 suppliers
$45.00/100mg

1360599-43-4
24 suppliers
inquiry