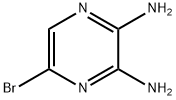
5-BROMO-PYRAZINE-2,3-DIAMINE synthesis
- Product Name:5-BROMO-PYRAZINE-2,3-DIAMINE
- CAS Number:89123-58-0
- Molecular formula:C4H5BrN4
- Molecular Weight:189.01
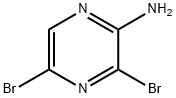
24241-18-7

89123-58-0
The general procedure for the synthesis of 5-bromo-2,3-diaminopyrazine from 2-amino-3,5-dibromopyrazine was as follows: step 1: 2-amino-3,5-dibromopyrazine (3 g, 11.64 mmol) was suspended in a 25% aqueous ammonia solution (15 mL) in a sealed tube and the reaction was heated for 16 h at 110 °C. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction mixture was partitioned between ethyl acetate and water (3 x 50 mL). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography with 15% ethyl acetate-hexane mixed solvent as eluent to afford 5-bromo-2,3-diaminopyrazine as a yellow solid (2.1 g, 93% yield). Mass spectrum (electrospray ionization) m/z: 189 ([M+H]+).

24241-18-7
439 suppliers
$6.00/5g

89123-58-0
122 suppliers
$13.00/1g
Yield: 93%
Reaction Conditions:
with ammonium hydroxide in water at 110; for 16 h;sealed tube;
Steps:
54.1
Example 54; Step 1; A suspension of 1 (3 g, 11.64 mmol) in 25% aq. NH4OH (15 mL) in sealed tube was heated at 110° C. for 16 h. After completion of reaction (reaction progress was monitored by TLC), the reaction mixture was partitioned between ethyl acetate-water (3×50 mL). The combined extracts were washed with saturated brine, dried and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel eluted with 15% ethyl acetate-hexane to afford 2 as a yellow solid (2.1 g, 93%). MS m/z (ES): 189 (M+H)+.
References:
de Vicente Fidalgo, Javier;Hermann, Johannes Cornelius;Lemoine, Remy;Li, Hongju;Lovey, Allen John;Sjogren, Eric Brian;Soth, Michael US2011/59118, 2011, A1 Location in patent:Page/Page column 81-82