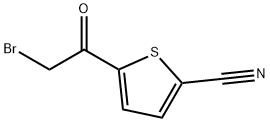
5-(bromoacetyl)thiophene-2-carbonitrile synthesis
- Product Name:5-(bromoacetyl)thiophene-2-carbonitrile
- CAS Number:496879-84-6
- Molecular formula:C7H4BrNOS
- Molecular Weight:230.08
88653-55-8
112 suppliers
$42.95/0.5g

496879-84-6
39 suppliers
inquiry
Yield:-
Reaction Conditions:
with bromine in 1,4-dioxane;diethyl ether; for 2 h;
Steps:
32 Preparation 32;3 5- (2-Bromo-1-hydroxyethyl)thiophene-2-carbonitrile
To a solution of 2-acetyl-5-cyanothiophene (1.5 g) in 20 mL of p-dioxane/ethyl ether, 1: 2, v/v) is introduced bromine (0.5 mL). After 2 h, ice water (30 mL) is added. The solid precipitates are collected by filtration and washed with water to afford 1.4 g of a white solid. The filtrate is allowed to stand overnight to give a second crop of 0. 86 g of white product for a total yield of 2.26 g 5- (bromoacetyl) thiophene-2-carbonitrile. Physical CHARACTERISTICS. 1H NMR (DMSO-D6) 8 8.16, 8.11, 4.94 ; MS (ESI-) M/Z 230 (M-H) . NABI-L (0.46 g in 5 ML of water) is added to a suspension of 5- (bromoacetyl)- thiophene-2-carbonitrile (1.85 g) in methanol (50 mL) cooled TO-10 °C. AFTER 10 MIN, 48% aq. HBr is added to adjust the pH to 3. The reaction mixture is concentrated to approximately 25 mL before water (30 mL) is added. The mixture is extracted with dichloromethane (3 x 40 ML). The organic phases are combined, washed with brine, and dried over MGS04. Removal of the solvent gave 1.6 g of the title compound as an orange oil. Physical CHARACTERISTICS. 1H NMR (DMSO-D6) No. 7.86, 7.23, 6.67, 5.17, 3.81, 3.68 ; MS (ESI-) M/Z 232 (M-H)-.
References:
WO2004/106345,2004,A2 Location in patent:Page 48-49

88653-55-8
112 suppliers
$42.95/0.5g
![2-Thiophenecarbonitrile, 5-[1-hydroxy-2-(methylamino)ethyl]-](/CAS/20210305/GIF/556025-60-6.gif)
556025-60-6
1 suppliers
inquiry

496879-84-6
39 suppliers
inquiry