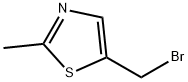
5-(Bromomethyl)-2-methylthiazole synthesis
- Product Name:5-(Bromomethyl)-2-methylthiazole
- CAS Number:838892-95-8
- Molecular formula:C5H6BrNS
- Molecular Weight:192.08
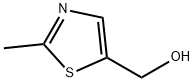
56012-38-5

838892-95-8
Step 2: Synthesis of 2-methyl-5-hydroxymethylthiazole At room temperature, 2-methyl-5-hydroxymethylthiazole (12 g, 0.1 mol) was dissolved in dichloromethane (500 mL) and the resulting solution was cooled to 0-5 °C in an ice water bath. Subsequently, triphenylphosphine (52 g, 0.2 mol) and carbon tetrabromide (66 g, 0.2 mol) were added to the cooled mixture in batches. After the addition was completed, the reaction mixture was allowed to warm up naturally to room temperature and stirred continuously at this temperature for 2 hours. Upon completion of the reaction, the mixture was concentrated and purified by silica gel column chromatography (eluent ratio of petroleum ether:ethyl acetate = 2:1), resulting in a yellow solid product (3.3 g, overall yield in two steps: 15%).

56012-38-5
66 suppliers
$45.00/5mg

838892-95-8
46 suppliers
$45.00/10mg
Yield:838892-95-8 3.3g
Reaction Conditions:
with carbon tetrabromide;triphenylphosphine in dichloromethane at 0 - 20; for 2 h;
Steps:
2 Step 2:
Step 2:
Synthesis of 2-methyl-5-bromomethylthiazole
At room temperature, 2-methyl-5-hydroxymethylthiazole (12g) was dissolved in methylene chloride (500mL), and the resulting solution was cooled to 0-5°C in an ice-water bath.
To the cooled mixture were added successively and in batches triphenylphosphine (52g, 200mmol) and carbon tetrabromide (66g, 200mmol).
After the completion of the addition, the reaction mixture was naturally warmed to room temperature, stirred for 2 hours at room temperature, concentrated, and purified by a silica gel column chromatography (petroleum ether:ethyl acetate=2:1) to produce a yellow solid (3.3g, the total yield for two steps: 15%).
References:
EP3181554,2017,A1 Location in patent:Paragraph 0092
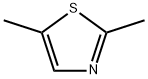
4175-66-0
135 suppliers
$21.00/1g

838892-95-8
46 suppliers
$45.00/10mg
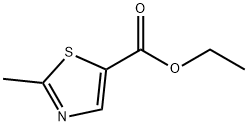
79836-78-5
138 suppliers
$13.43/250mgs:

838892-95-8
46 suppliers
$45.00/10mg