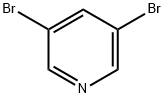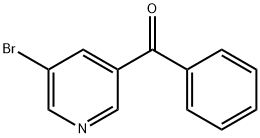
(5-BroMopyridin-3-yl)(phenyl)Methanone synthesis
- Product Name:(5-BroMopyridin-3-yl)(phenyl)Methanone
- CAS Number:59105-50-9
- Molecular formula:C12H8BrNO
- Molecular Weight:262.1
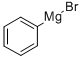
100-58-3
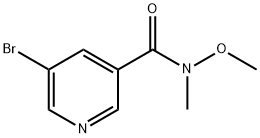
183608-47-1

59105-50-9
A solution of 5-bromo-N-methoxy-N-methylnicotinamide (400 mg, 1.63 mmol) dissolved in tetrahydrofuran (10 mL) was cooled to -20 °C and slowly added dropwise to a solution of phenylmagnesium bromide (1 M, 4.9 mL) in tetrahydrofuran under argon protection. The reaction mixture was kept at -20 °C for 1 h. The reaction was then slowly warmed up to room temperature and continued for 4 hours. Upon completion of the reaction, the reaction was quenched with saturated aqueous sodium bicarbonate solution and the tetrahydrofuran was removed by distillation. The mixture was extracted with ethyl acetate and the organic phase was dried with anhydrous sodium sulfate, filtered and the solvent evaporated. The residue was purified by silica gel column chromatography to afford the target product (5-bromopyridin-3-yl)(phenyl)methanone (260 mg, 61% yield).
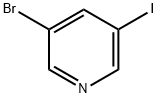
233770-01-9
199 suppliers
$8.00/250mg
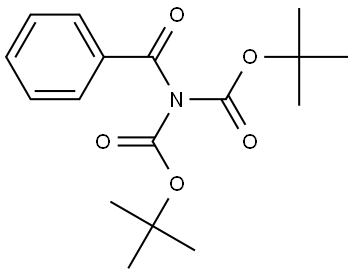
135364-97-5
0 suppliers
inquiry

59105-50-9
26 suppliers
inquiry
Yield:59105-50-9 62%
Reaction Conditions:
Stage #1: 3-bromo-5-iodopyridinewith TurboGrignard in tetrahydrofuran at -20; for 0.833333 h;Inert atmosphere;Schlenk technique;
Stage #2: tert-butyl benzoyl(tert-butoxycarbonyl)carbamate in tetrahydrofuran at -20 - 20; for 2.5 h;Inert atmosphere;Schlenk technique;chemoselective reaction;
References:
Li, Guangchen;Szostak, Michal [Chemistry - A European Journal,2020,vol. 26,# 3,p. 611 - 615] Location in patent:supporting information
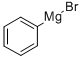
100-58-3
195 suppliers
$18.00/10ml

183608-47-1
45 suppliers
$110.00/500mg

59105-50-9
26 suppliers
inquiry
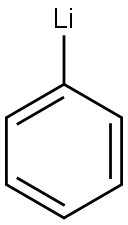
591-51-5
134 suppliers
$123.00/50ml

183608-47-1
45 suppliers
$110.00/500mg

59105-50-9
26 suppliers
inquiry

233770-01-9
199 suppliers
$8.00/250mg
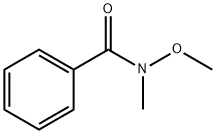
6919-61-5
135 suppliers
$14.00/1g

59105-50-9
26 suppliers
inquiry